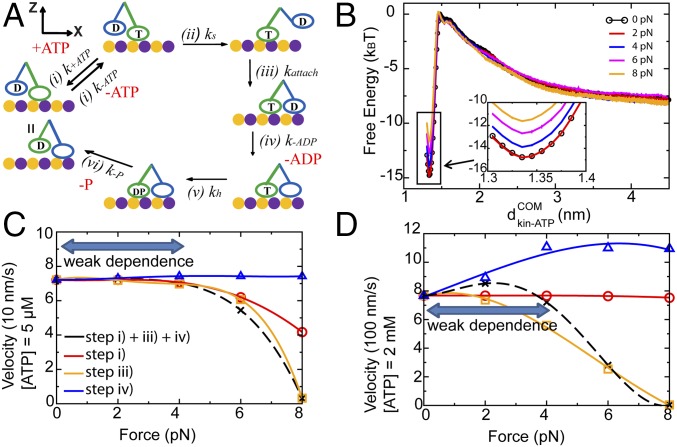
Fig. 1.
The chemomechanical cycle of a single double-headed kinesin under an external force. (A) A schematic diagram of the chemomechanical cycle of a single double-headed kinesin on an MT. T represents an ATP-bound motor head. D represents an ADP-bound motor head. DP represents an ADPP-bound motor head; k represents the reaction rate of each step in a cycle. (B) The free energy profile of binding/unbinding between the ATP and the leading head (LH) in step i at 300 K with external loading forces of F = 0, 2, 4, 6, and 8 pN separately. represents the distance between the center of mass of ATP and that of the LH. The free energy profile in the bound state ( = 1.34 nm) is zoomed in and shown in Inset. (C and D) Numerically calculated velocity of single kinesin as a function of external loading forces at (C) 5 μM ATP and (D) 2 mM ATP. The black dashed lines represent the calculated velocity when steps i, iii, and iv are all force-dependent. The profile shows a weak dependence on small forces. The red solid lines represent the calculation assuming that only step i is force-dependent. For other steps, we assume that they are force-independent. The orange solid lines represent the calculation assuming that only step iii is force-dependent. The blue solid lines represent the calculation assuming that only step iv is force-dependent. Steps i (ATP release) and iii (attachment) cause a slow decrease in the velocity under loading forces. They serve as important steps to resist small forces. Error bars are shown in B.