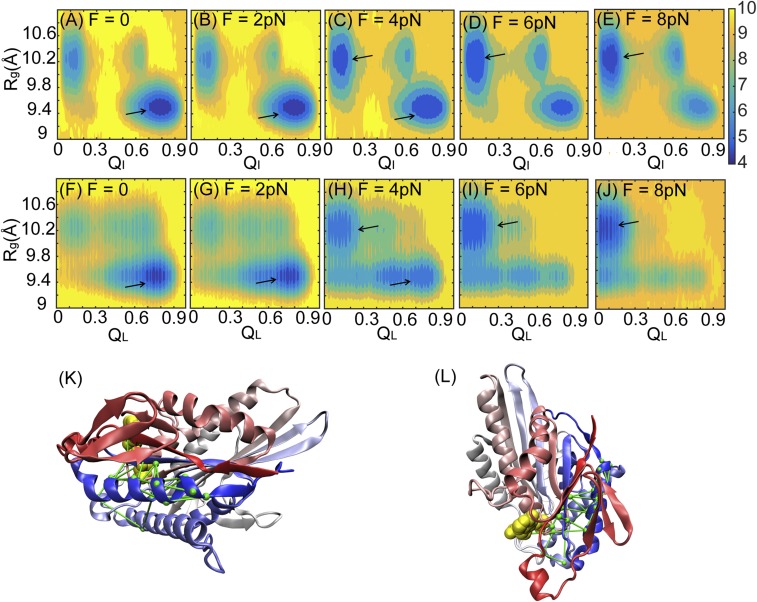
Fig. 2.
Conformational changes of a single double-headed kinesin under an external force. (A–E) 2D free energy as a function of the radius of gyration of the ATP binding pocket, Rg, and the fraction of native contact formation between a single kinesin and ATP, QI, at loading forces of F = 0, 2, 4, 6, and 8 pN separately. (F–J) 2D free energy as a function of Rg and the fraction of native contact formation between the leading head and the connecting neck linker, QL, at loading forces of F = 0, 2, 4, 6, and 8 pN separately. The free energy is colored in units of kBT at T = 300 K. Black arrows point to the basin of the low free energy from each panel. (K and L) Structure of a motor head and its connecting neck linker with a network of lines from the DCA. The kinesin motor head is colored from red (N terminus) to blue (C terminus) and shown from the front view (K) and the top view (L). ATP is colored in yellow.