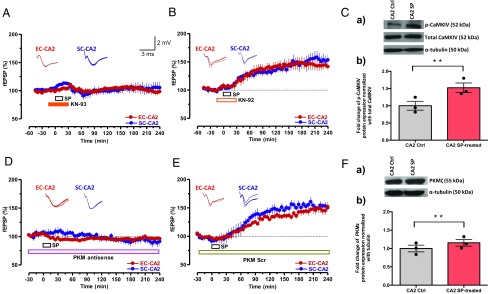
Fig. 4.
PKMζ and CaMKIV are required for SP-induced plasticity and associativity in CA2 neurons. (A) The CaMKIV inhibitor KN-93 (10 µM) and SP were coapplied as indicated by horizontal bars in the graph (total of 45 min). The coapplication prevented the induction of SP-mediated potentiation in both synaptic inputs (n = 8). (B) Experimental design and drug application similar to that in A except that the nonactive drug KN-92 was used (n = 8). (C, a and b) Western blot analysis showed a significant increase of CaMKIV protein phosphorylation in the CA2 region after SP treatment compared with the respective control. The significant difference between the groups (CA2 control vs. CA2 SP-treated) is indicated by **P < 0.01 (from three biological replicates). Individual data points of fold change are represented within the bar graphs. (D) Preincubation (1.5 h) and continuous application of PKMζ antisense oligodeoxynucleotides (20 μM) for as long as 240 min prevented SP-induced fEPSP potentiation (n = 7). (E) Experimental design similar to that in D except that a scrambled version of PKMζ antisense oligodeoxynucleotides was applied (n = 7). Representative fEPSP traces 15 min before (closed line), 60 min after (dotted line), and 180 min after (hatched line) SP application are depicted. Calibration bars for fEPSP traces are 2 mV/3 ms. (F, a and b) Western blot analysis of PKMζ protein expression also showed a significant up-regulation in CA2 region after SP treatment compared with control. The significant difference between the groups (CA2 control vs. CA2 SP-treated) is indicated by **P < 0.01 (from three biological replicates). Individual data points of fold change are represented within the bar graphs.