Figure 1.
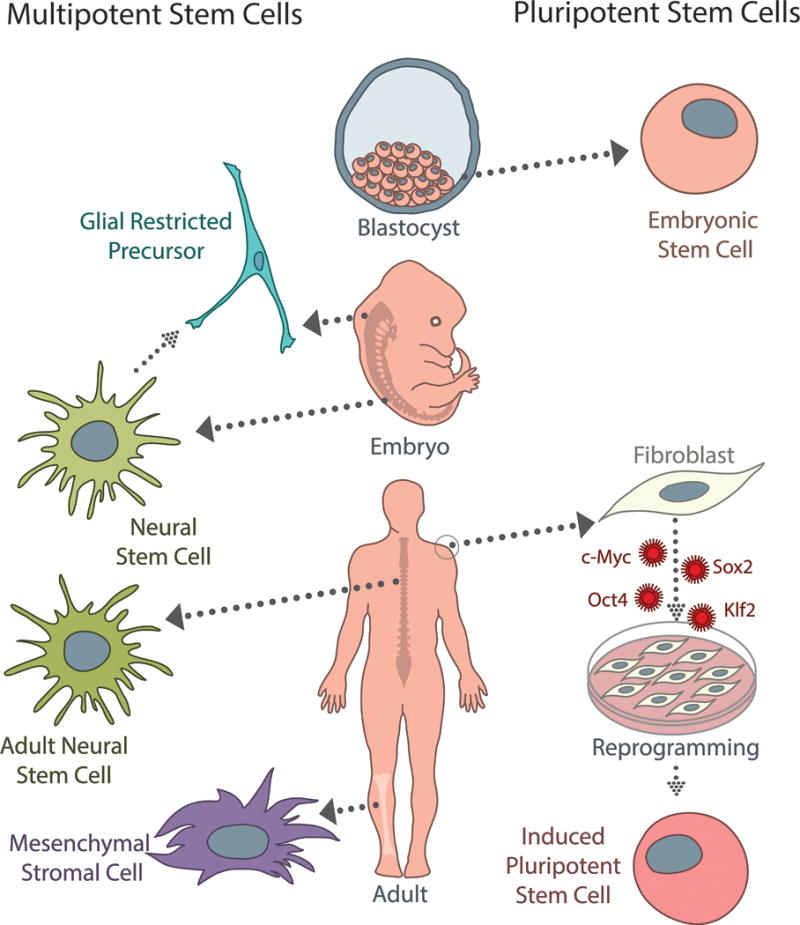
There are several sources of multipotent (left) and pluripotent (right) stem cells currently used for spinal cord injury. Neural stem cells (NSCs) can be derived from fetal or adult tissue, and are capable of differentiating into neurons, oligodendrocytes, and astrocytes. While not typically considered stem cells, glial-restricted precursors (GRPs) are a commonly studied, tri-potent population that can be isolated from neural stem cells or fetal tissue directly. GRPs differentiate into oligodendrocyte progenitor cells and two types of astrocytes. Mesenchymal stromal cells (MSCs) are an appealing population clinically because they can be isolated from adult bone marrow or peripheral blood; however, while they are capable of differentiating into a wide variety of cells types, the efficacy of neuronal differentiation is a specific concern for SCI treatment. Embryonic stem cells (ESCs) are a pluripotent population, which can give rise to cell types from all three germ layers; however, because they are derived from the inner cell mass of early blastocysts, ethical considerations limit their clinical potential. Induced pluripotent stem cells (iPSCs) can be generated from adult somatic cells (fibroblasts, melanocytes, cord or peripheral blood cells, adipose stem cells, etc.) by several different reprogramming methods using the Yamanaka factors (c-Myc, Sox2, Oct4, Klf2). While induction and reprogramming efficiencies remain a concern, iPSCs represent an autologous, patient-specific population that has significant clinical potential as the field progresses.
