Figure 2.
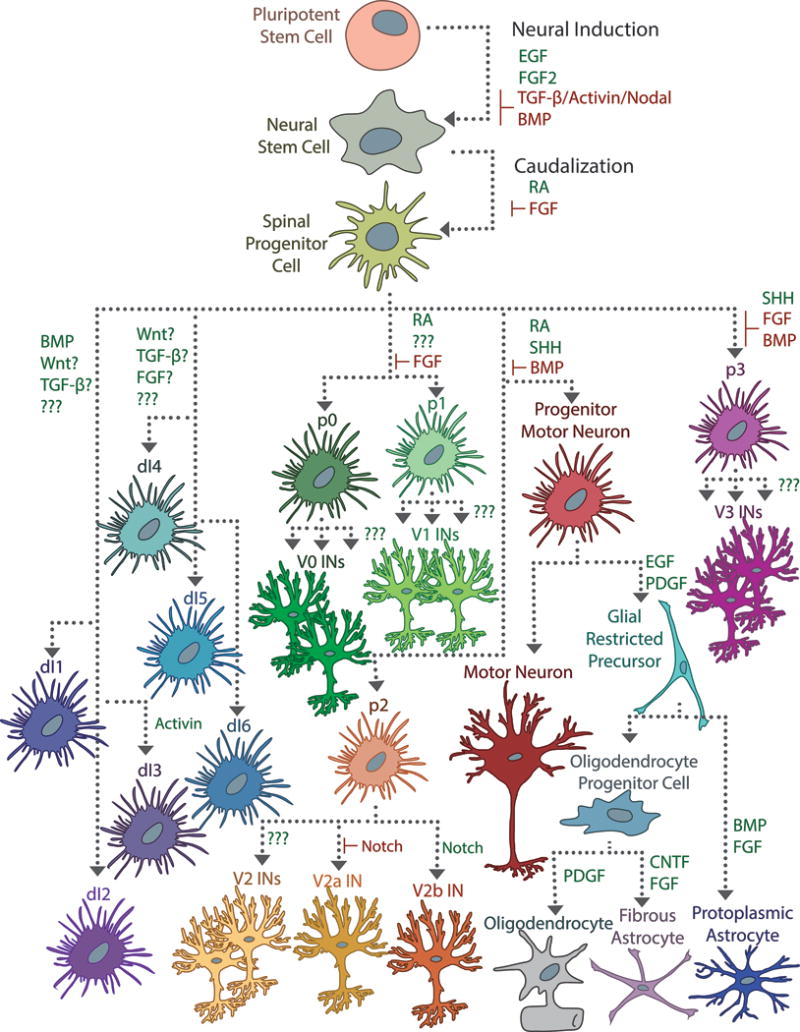
Directing differentiation of pluripotent stem cells is traditionally achieved by triggering the signaling cascades responsible for cell fate determination. Neural induction from pluripotent cells typically begins with conversion into a neural stem cell (NSC), which may involve stimulating EGF and FGF2 signaling and inhibiting BMP and TGF-β/Activin/Nodal signaling. Subsequent exposure with RA caudalizes the NSCs and ensures a spinal identity. A complex interplay of signaling events is responsible for the dorsoventral patterning of the spinal cord, made more opaque by the importance of exposure duration and cell-cell signaling events. In the developing spinal cord, Shh from the notochord and floor plate interact with RA to induce differentiation of ventral progenitor cells (p0–p3, pMN), which mature into motor neurons, oligodendrocytes, astrocytes, and a variety of ventral interneuron populations. A combination of BMP, Wnt, FGF, and TGF-β signaling induces differentiation of the dorsal progenitor populations (dI1–dI6), but the precise mechanisms are poorly understood. Some specific factors that influence progenitor subspecialization have been determined experimentally in vitro, especially with regards to glial-restricted progenitor cells (GRPs), but for most interneuron populations, these are unknown.
