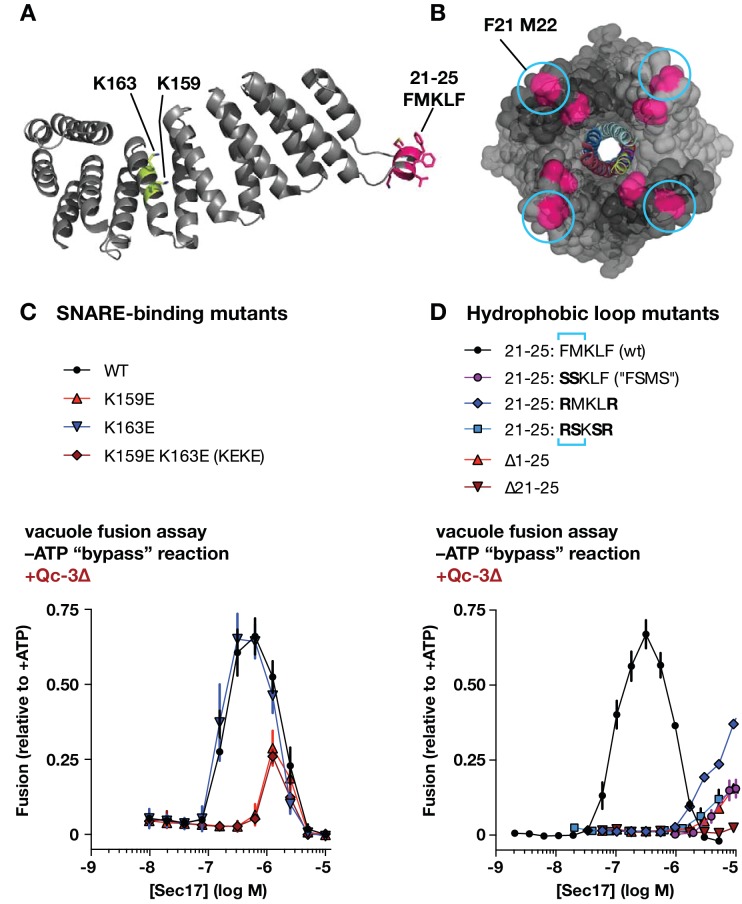
Figure 5. Effects of Sec17 mutations on stimulation of fusion.
(A) Locations of Sec17 mutations. The diagram is a rendering of PDB 1QQE (Rice and Brunger, 1999). The N-terminal hydrophobic loop is shaded magenta. Two highly conserved lysine residues are shaded green. (B) Position of Sec17 hydrophobic loop relative to SNARE core complex. Rendering shows a SNARE complex with four bound α-SNAP molecules, from the perspective of the membrane-proxiimal SNARE domain C-termini. Hydrophobic loop residues are colored magenta. The two residues homologous to those mutated in Sec17-FSMS are circled. The rendering is based on PDB 3J96 (Zhao et al., 2015). (C and D) Ability of Sec17 mutants to rescue Qc-3∆ trans-SNARE complexes in vitro. Vacuole fusion reactions were assembled in the gain-of-function –ATP (‘bypass’) configuration (Figure 3—figure supplement 1, reaction ii), with 75 nM Qc-3∆ and the indicated concentrations of Sec17 or its mutants. Fusion is normalized relative to the signals from standard ATP-driven reactions without added Sec17. Each point denotes the mean ± s.e.m. of three independent experiments.