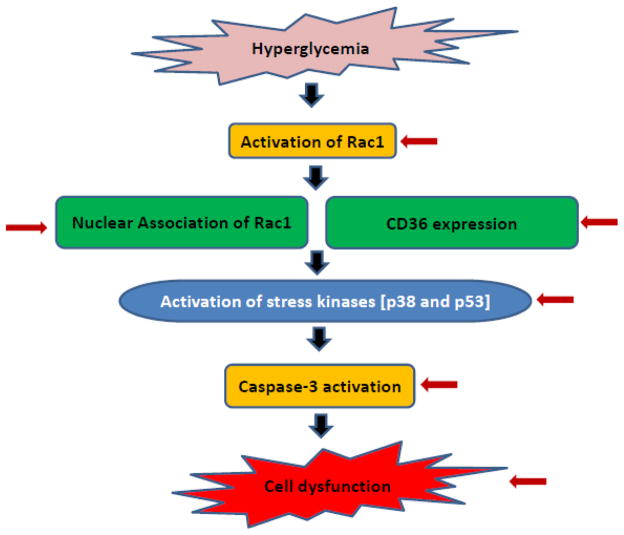
Figure 9. A proposed model for metabolic stress induced dysfunction of pancreatic islet β-cells: Reversal by metformin.
Based on the data accrued thus far, we propose that exposure of pancreatic islet β-cells to metabolic stress conditions [e.g., glucotoxicity results in functional inactivation of prenyltransferases, and consequently, a significant accumulation of unprenylated Rac1 occurs. Our findings suggest a paradoxical stimulation of G protein activity even at the height of inhibition of requisite prenylation. We presented data herein to suggest a significant association of unprenylated, but active [GTP-bound] Rac1 with the nuclear fraction in a variety of insulin-secreting cells under glucotoxic conditions. This, in turn, promotes phosphorylation of pro-apoptotic proteins/factor, including p53 [15]. Along these lines, we also demonstrated that constitutively activated Rac1 promotes activation of Nox2 resulting in the generation of oxidative stress and activation of downstream signaling kinases including p38MAPK. These events singly, or in combination, promote caspase activation, nuclear lamin degradation leading to cell dysfunction. Note that specific signaling events/steps that are regulated by metformin [current studies] are indicated by red arrows.