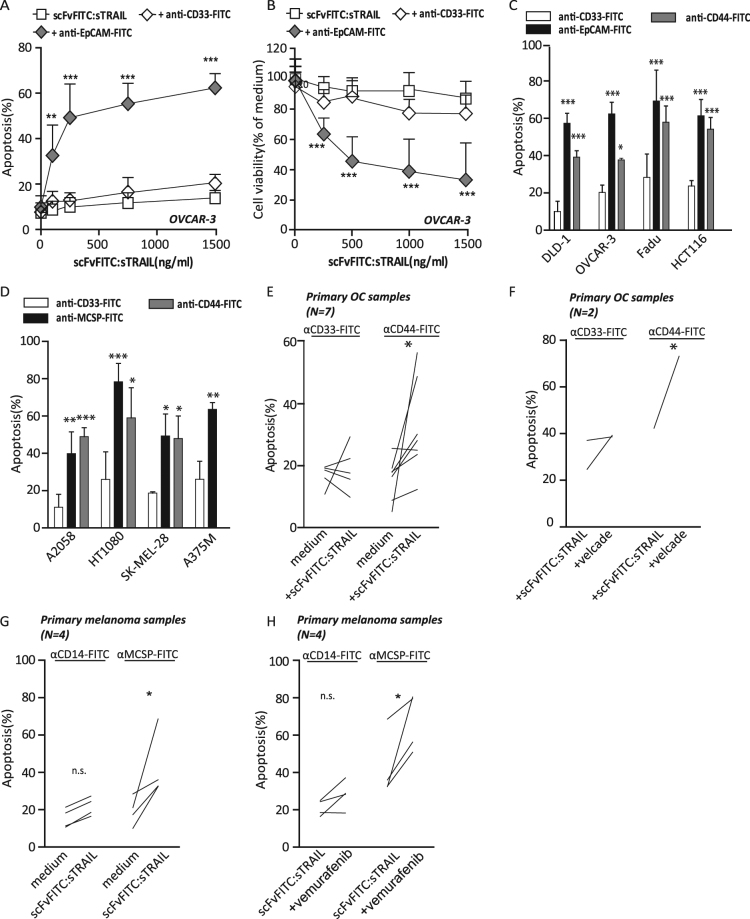
Figure 2.
scFvFITC:sTRAIL-mediated apoptosis in solid tumour cells pretargeted with FITC-labelled MAbs. (A) OVCAR-3 cells were incubated with anti-CD33-FITC (isotype control) or anti-EpCAM-FITC followed by treatment with an increasing dose of scFvFITC:sTRAIL (up to 1.5 µg/ml) for 24 h. Apoptosis was determined by Annexin V/PI staining. (B) In the same experimental setting as (A), cell viability of OVCAR-3 cells was determined by MTS assay after 72 h treatment. (C) In an extended analysis, a panel of carcinoma cells was pretargeted with anti-CD44-FITC or anti-EpCAM-FITC followed by scFvFITC:sTRAIL treatment (1.5 µg/ml). (D) A panel of MCSPpos cancer cells were incubated with anti-MCSP-FITC or anti-CD44-FITC followed by treatment of scFvFITC:sTRAIL (1.5 µg/ml). (E) Seven primary patient-derived ovarian samples were pretargeted with anti-CD33-FITC (isotype control) or anti-CD44-FITC followed by treatment of scFvFITC:sTRAIL (1.5 µg/ml). (F) Two pretargeted primary OC samples with co-treated with scFvFITC:sTRAIL and Velcade (5 µM) (G); Four primary patient-derived melanoma samples were pretargeted with anti-MCSP-FITC followed by treatment of scFvFITC:sTRAIL(1.5 µg/ml). (H) Anti-MCSP-FITC-pretargeted primary melanoma samples (N = 4) were co-treated with scFvFITC:sTRAIL and vemurafenib (10 μM). Apoptosis in all experiments was determined by Annexin V/PI staining. All graphs represent mean + SD. Statistical analysis was performed using two-way ANOVA (*p < 0.05, **p < 0.01, ***p < 0.001, n.s. not significant).