Abstract
Background:
Psoriasis is a T cell-mediated autoimmune disease in patients with elevated levels of proinflammatory cytokines belonging mainly to the Th1 pathway. We investigated whether treatment of psoriasis patients with methotrexate (MTX), along with micronutrients, modulated mRNA expression of Th1 and Th2 components and whether expression of these components correlated with psoriasis severity.
Methods:
Thirty plaque-type psoriasis patients with Psoriasis Area and Severity Index (PASI) scores greater than 10 were recruited; these were 15 non-micronutrients taking- (NMT) patients treated with MTX daily (0.2-0.3 mg/kg/week), and 15 micronutrients taking- (MT) patients treated with MTX plus a micronutrient supplement daily, for 12 weeks. Blood samples were collected immediately before treatment (baseline) and after 12 weeks of treatment. Taqman quantitative real-time polymerase chain reaction (qPCR) was applied to analyze the expression of the Th1 components T-bet, interleukin-12 (IL-12), and interferon-gamma (IFN-Υ), and the Th2 components GATA-3 and interleukin-4 (IL-4). Disease severity was measured using the PASI scoring system.
Results:
Significant clinical improvement in the MT group coincided with significant down-regulation of Th1 and up-regulation of Th2 markers (P<0.05). With respect to the PASI-75, (defined as a 75% or greater reduction in the PASI score) cut-off point, expression of IFN-γ in the MT group with PASI scores above 75 was significantly less than that of patients in the NMT group (P=0.05). Also, GATA3 and IL-4 mRNA expression in the MT group with PASI scores greater than above 75 was significantly greater than that of patients in the NMT group (P=0.05 and 0.04, respectively)
Conclusion:
Based on significant attenuation of the PASI score, which correlated with upregulation of Th2 pathway markers in the MT group, we recommend administration of micronutrients combined with MTX for psoriasis patients. Our results contribute to a better understanding of methotrexate immunepathogenesis mechanisms and their correlations to clinical responses in psoriasis.
Key Words: Cytokine, Gene expression, Methotrexate, Micronutrients, Psoriasis, Transcription factor
Introduction
Psoriasis is a common chronic inflammatory skin disease characterized by epidermal cell hyperproliferation and turnover with an activated mononuclear cell infiltrate into the underlying dermis (1, 2). Psoriasis lesions develop through an immune deregulation pathway involving overexpression of pro-inflammatory cytokines produced by T helper (Th) 1 cells and dysfunction of Th2 cells (3-5). The pathogenesis of psoriasis is not clear, but it is believed to be induced by autoreactive interferon-gamma (IFN-Υ) producing Th1 lymphocytes, which regulate other cellular reactions, resulting in hyper-proliferation of keratinocytes with associated inflammation and dermal proliferation of small vessels (6). The role of Th1 cell differentiation and Th1 cytokines in the development and maintenance of psoriasis has been elucidated through increased activation of CD4(+) T lymphocytes and systemic and local overexpression of the pro-inflammatory cytokines IFN-Υ, IL-6, and tumor necrosis factor alpha (TNF- α) (7). The role of complementary or alternative medicine (CAM) in psoriasis management to reduce flare-ups, scales, and erythema has been neglected (8, 9). Some treatments have attempted to tweak the Th1/Th2 pathway with micronutrient supplements (10-12). Therefore, clinical trials to evaluate the Th1/Th2 imbalance in psoriasis are needed. Methotrexate (MTX), the cost-effective gold-standard treatment for moderate to severe psoriasis, acts as both an immune-modulatory and antimetabolite agent (13-15). Methotrexate was originally thought to not influence the Th1/Th2 balance but it has been recently reported to modulate the immune status towards Th2 dominance in rheumatoid arthritis by decreasing expression of the interleukin- (IL) 12 receptor and IFN-Υ, and increasing expression of IL-4 (16, 17). The mechanisms that control the Th1/Th2 balance are not yet fully understood, but the transcription factors T-bet and GATA-3 have been shown to influence Th cell differentiation (18-20). T-bet, a member of the Tbr1 sub-family of T-box genes, plays an important role in Th1 differentiation. T-bet controls the expression of IFN-Υ, a Th1-specific cytokine, and inhibits the production of IL-4, a Th2-specific cytokine, generating the shift from Th2 to Th1 cell dominance (20-22). In addition, GATA-3, the specific Th2 pathway transcription factor, promotes Th2 differentiation and Th2 cytokine expression (23). The relative expressions of T-bet and GATA-3, which affect the Th1 and Th2 pathways, has been implicated in several immunological diseases including atopic dermatitis, rheumatoid arthritis, and psoriasis (24-26).
It seems the pathogenesis of psoriasis includes the immune responses of both Th1 and Th2 cells, although how this Th1/Th2 skewing leads to psoriasis is not fully understood. To our knowledge, the effect of co-consumption MTX and micronutrients on the expression Th1 and Th2 components in peripheral blood mononuclear cells (PBMCs) of psoriasis patients has not been investigated.
In this report, using quantitative reverse transcriptase polymerase chain reaction (qPCR), we examined the expression of GATA-3, T-bet, IL-4, IL-12, and IFN-Υ in PBMCs of psoriasis patients in two treatment modalities including MTX only and MTX plus micronutrients. We also evaluated the correlations between clinical responses and expression of the Th1 and Th2 factors.
Materials and Methods
This case-control study was approved by the Ethics Committee of Mashhad University of Medical Sciences (MUMS), Mashhad, Iran, and under the registry of Iranian Registry of Clinical Trials; IRCT: 2014012016275N1. Written informed consents were acquired from all participants.
Study participants and treatment regimen
Thirty plaque-type psoriasis patients included 18 men and 12 women with a mean age ± standard deviation (SD) of 38.67 ± 10.81 years and a range of 21–59 years. Patients with Psoriasis Area and Severity Index (PASI) scores greater than 10 were included according to the inclusion and exclusion criteria. Patients with the following criteria were excluded; type one diabetes mellitus, obesity with body mass index (BMI) > 25, cardiovascular disease, pregnancy, history of liver disease, or consumption of alcohol or alcoholic beverages during the study. Those who wanted to leave the study for any reason, those who took methotrexate, those who received phototherapy within the previous two months, or those who self-consumed micronutrients or over-the-counter (OTC) medicines for psoriasis within the previous two months were excluded.
The non-micronutrient-taking (NMT) patients were considered as controls and the micronutrienttaking (MT) patients were considered as cases.
Subjects in both groups received one dose of MTX at 0.2-0.3 mg/kg/week for 12 weeks (27). Subjects in the MT group also received one tablet of micronutrient supplement during the 12-week study (Immunace, Vitabiotics Ltd, London, UK). In addition, folic acid was given to all study subjects (5 mg once daily) except on the day of MTX consumption. Psoriasis severity was rated according to the Psoriasis Area and Severity Index (PASI) scoring system (28), at the beginning (baseline) and end of the study. Drug approval often depends on a 75% improvement in the baseline PASI score and known as a PASI-75 (28). Therefore, we considered PASI-75 as the clinical cut-off point and the clinical effectiveness indicator. Subjects who achieved PASI scores > 75% achieved greater clinical improvement than patients with PASI scores < 75%.
Furthermore, to identify probable confounding variables related to the expression of our genes of interest, age, age at disease onset, weight, body mass index (BMI), and dermatology quality of life index (DLQI) were assessed. The DLQI was recorded based on the DLQI questionnaire (29).
Isolation of PBMCs
Five ml of blood were collected from each subject into a vacutainer tube under sterile conditions between 08:30 and 10:30 a.m. at baseline and at the end of the trial. To isolate serum, blood samples were incubated at room temperature for 30 min and centrifuged at 2800 rpm for 20 min. Peripheral blood mononuclear cells (PBMCs) from the blood samples were isolated using Ficoll (Lymphosep, Biosera, UK). The isolated cells were centrifuged for 10 min at 971 g and washed 2x with phosphate buffered saline (PBS) (Sigma, Germany).
Quantitative real-time PCR (qPCR)
mRNA expression of T-bet, GATA3, IFN-Υ, IL-12, and IL-4 was measured by qPCR. Total RNA was isolated from PBMCs using Trizol (Roche Diagnostics GmbH, Mannheim, Germany). cDNA was synthesized using a Prime-ScriptTM, RT reagent kit (Takara Biotechnology, Otsu-Shiga, Japan) according to the manufacturer's instructions. Quantitative real-time PCR was performed on the Rotor-Gene Q (Qiagen Hilden, Germany) using Taqman Master Mix (Primemix EXTaqTM, Takara Biotechnology, Otsu-Shiga, Japan) with appropriate primers and probes (Table 1). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an endogenous control for sample normalization. Based on the expression of target genes normalized to GAPDH, we calculated the relative quantification delta-delta Ct and the results are presented as fold change.
Table 1.
Studied primers and gene probe sequences used in quantitative real-time PCR
| Gene | Accession No. | Sequence 5´-3´ (position) | |
|---|---|---|---|
| IL-4 | AM937235.1 | Forward | CGGCTCGACAGGAACCTCT |
| Reverse | TCCAAGAAGTTTTCCAACGTACTCT | ||
| Probe | FAM-CGGGCTTGAATTCCTGTCCTGTGAAG-TAMRA | ||
| IL-12 | AF101062.1 | Forward | GAGGGCCGTCAGCAACAT |
| Reverse | ATGATCAATCTCTTCAGAAGTGCAA | ||
| Probe | FAM-CTCCAGAAGGCCAGACAAACTCTAGAATTTTACC-TAMRA | ||
| GATA3 | NM_002051 | Forward | TTCCCCAAGAACAGCTCGTT |
| Reverse | GGCTCAGGGAGGACATGTGT | ||
| Probe | FAM-AACCCGGCCGCCCT- TAMRA | ||
| T-bet | AF241243.2 | Forward | ACACGCATATCTTTACTTTCCAAGAA |
| Reverse | TCAGCTGAGTAATCTCGGCATTC | ||
| Probe | FAM-CCCAGTTCATTGCCGTGACTGCC-TAMRA | ||
| IFN-γ | BC070256.1 | Forward | CTAATTATTCGGTAACTGACTTGA |
| Reverse | ACAGTTCAGCCATCACTTGGA | ||
| Probe | FAM-TCCAACGCAAAGCAATACATGAAC-TAMRA | ||
| GAPDH | NM_001256799.2 | Forward | AGCCGGGCATGTTCTTCAAC |
| Reverse | AGGGAGCTTCACGTTCTTGTAT | ||
| Probe | FAM- CGGAGCTGGACCTGACCTACGGCA -TAMRA | ||
Statistical analysis
Data were analyzed by SPSS software version 16. Descriptive data were summarized as means with standard errors of means (SEMs). The Mann-Whitney test or the Independent T-test was used to compare expression of transcription factors and cytokines between the MT and NMT groups. Pearson’s correlation test was used to evaluate the correlation between PASI scores and the Th1 and Th2 markers. Spearman’s correlation test was performed to evaluate the correlation of variables including age, age at disease onset, weight, BMI, DLQI, and the expression of T-bet, GATA3, IFN-Υ, IL-12, and IL-4. P values less than or equal to 0.05 were considered significant.
Results
In this study, 30 psoriasis patients were divided into two groups; the NMT group included 9 males and 6 females, 38.80±11.23 years old, and the MT group included 9 males and 6 females, 38.53 ± 10.77 years old. The two groups were similar in gender distribution and age (P=0.97). In addition, subjects in the NMT and MT groups had similar weights (67.5 ± 5.37 vs. 61.90 ± 12.87) and BMIs (23.82±2.34 vs. 21.75±2.40) (P>0.05). The NMT and MT groups also had similar PASI scores (30.23±10.87 vs. 31.80±10.57) before the study (P=0.69), while after the 12-week study, the PASI score of the MT group was significantly less than that of the NMT group (5.50±3.82 vs. 10.86± 9.84, P=0.04). Six subjects of the NMT and 11 subjects of the MT group reached PASI-75 at week 12 (P=0.05). Variations of DLQI among NMT and MT participants were similar both before (15.13± 2.85 vs. 15.33±3.30) and after the experiment (9.73± 1.94 vs. 8.53 ± 2.97), respectively (P >0.05).
Modification of Th1 Pattern
Figure 1(Fig. 1) shows the relative expression of IFN-Υ, IL-12, and T-bet. mRNA expression of IFN-Υ, IL-12, and T-bet was significantly greater in the NMT than in the MT group (Fig. 2A, P = .01, .05, and .05, respectively). Overall correlations between changes in PASI scores from baseline to the end of the study and expression of the Th1 pathway markers IFN-Υ, IL-12, and T-bet are presented in Figure 2(Fig. 2). The statistical analysis showed that there were not any significant correlations between expression of any of the markers and clinical responses. Table 2 shows the statistical analyses relating expression of the Th1 markers of the MT and NMT groups with respect to the PASI-75 scores. Expression of all the Th1 markers was greater in the NMT than in the MT group. Expression of IFN-Υ in subjects in the MT group with PASI scores above 75, indicating greater clinical improvement, was significantly less than that in the NMT group (P < 0.05). Although this point was presented for IL-12 and T-bet, the difference between the MT and NMT groups was not significant (Table 2, P > 0.05). To identify the confounding variables, we compared the correlation of clinical and demographic variables with cytokine gene expression during the study (Table 3). No significant correlations were observed between IFN-Υ, IL-12, T-bet, IL-4, or GATA3 expression and age, age at disease onset, weight, BMI, or DLQI (P > 0.05).
Fig. 1.
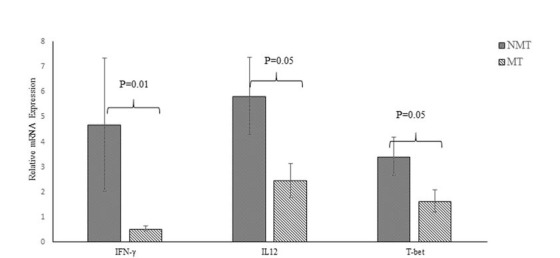
Relative mRNA expression of IFN-γ, IL-12, and T-bet in the NMT and MT groups. P values for each expression pair are shown. NMT; non-micronutrients takers, MT; micronutrients takers
Fig. 2.
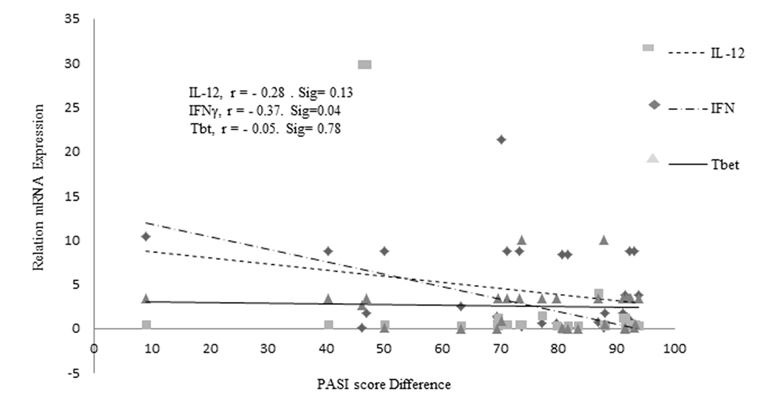
Correlation of Th1 marker gene expression and Psoriasis Area and Severity Index (PASI). r; Regression, Sig: Significant level
Table 2.
Evaluation of gene expression of Th1 and Th2 cytokines and PASI-75 as cut off point between NMT and MT groups.
| Pathway | Gene | PASI group | NMT, Mean ± SE | MT, Mean ± SE | P-value |
|---|---|---|---|---|---|
| Th1 | IFN-Υ | PASI < 75% | 6.98 ± 4.32 | 0.61 ± 0.2 | **0.01 |
| PASI > 75% | 1.23 ± 0.20 | 0.51 ± 0.10 | *0.05 | ||
| IL-12 | PASI < 75% | 7.61 ± 2.20 | 2.38 ± 0.89 | *0.05 | |
| PASI > 75% | 3.16 ± 1.75 | 1.45 ± 0.35 | 0.85 | ||
| T-bet | PASI < 75% | 3.40 ± 0.92 | 1.71 ± 0.99 | 0.29 | |
| PASI > 75% | 3.46 ± 1.44 | 1.62 ± 0.52 | 0.16 | ||
| Th2 | IL-4 | PASI < 75% | 1.89 ± 0.57 | 7.04 ± 2.26 | *0.05 |
| PASI > 75% | 1.84 ± 0.64 | 10.45 ± 3.23 | *0.04 | ||
| GATA3 | PASI < 75% | 12.87 ± 7.58 | 18.83 ± 6.80 | 0.64 | |
| PASI > 75% | 3.37 ± 1.77 | 20.58 ± 7.79 | *0.05 |
NMT; non-micronutrients taker, MT; micronutrients taker, PASI; Psoriasis Area and Severity Index, PASI < 75% and PASI > 75% refer PASI scores less than and above than 75 respectively, SE; The standard error of the mean, means represent the relation ratio mRNA expression of related studied genes. The Independent T-test for was used for parametric data and the Mann-Whitney U test for nonparametric data. *P values less than 0.05 were considered statistically significant and **P values less than 0.01 were considered statistically very significant.
Table 3.
Correlation analysis of anthropometric variables (age, age at disease onset, weight, and BMI) and dermatology quality of life index (DLQI) with related Th1 and Th2 transcription factors and cytokines
| Cytokine Expression | ||||||
|---|---|---|---|---|---|---|
| IFN-Υ | IL-12 | T-bet | IL-4 | GATA3 | ||
| Age | Correlation coefficient | 0.23 | 0.18 | 0.21 | -0.20 | -0.17 |
| Significance (two-tailed) | 0.20 | 0.32 | 0.25 | 0.28 | 0.34 | |
| Age at onset | Correlation coefficient | -0.01 | 0.01 | 0.11 | 0.01 | 0.009 |
| Significance (two-tailed) | 0.92 | 0.94 | 0.55 | 0.94 | 0.94 | |
| Weight (Kg) | Correlation coefficient | -0.07 | -0.20 | 0.11 | 0.08 | -0.09 |
| Significance (two-tailed) | 0.70 | 0.27 | 0.54 | 0.65 | 0.61 | |
| BMI (Kg/m2) | Correlation coefficient | -0.17 | 0.01 | 0.08 | -0.23 | -0.15 |
| Significance (two-tailed) | 0.36 | 0.95 | 0.65 | 0.23 | 0.40 | |
| DLQI | Correlation coefficient | -0.08 | 0.02 | 0.14 | -0.07 | 0.03 |
| Significance (two-tailed) | 0.64 | 0.88 | 0.46 | 0.67 | 0.86 | |
BMI; Body Mass Index, Correlations were calculated with Spearman’s Rho and less than 0.05 was considered as significant level.
Modification of Th2 Pattern
Interleukin-4 and GATA3 expression were significantly greater in the MT than in the NMT group (Figure 3 (Fig. 3), P < 0.05). Correlation of expression of the Th2 pathway factors GATA3 and IL-4 and PASI score differences showed that variations in expression of these factors were not correlated significantly with each other (P > 0.05,Figure 4). Hence, improvement in clinical response was not significantly related to IL-4 and GATA3 expression (Fig. 4). With respect to the PASI-75 cut-off point, GATA3 and IL-4 mRNA expression were significantly greater in the MT group members with PASI scores above 75 than those in the NMT group (P = 0.05, P = 0.04, Table 2). Furthermore, no significant correlation was observed between GATA3 or IL-4 expression and age, age at disease onset, weight, BMI, or DLQI (P > 0.05, Table 3).
Fig. 3.
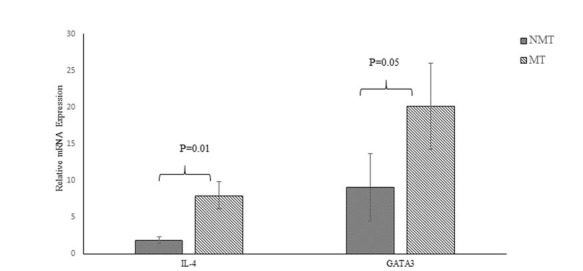
Relative mRNA expression of Th2 pathway factors IL-4 and GATA3 in NMT and MT groups. P values for each expression pair are shown. NMT; non-micronutrients takers, MT; micronutrients takers
Fig. 4.
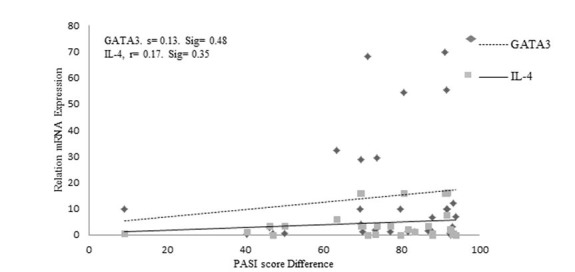
Correlation of expression of the Th2 pathway factors GATA3 and IL-4 and Psoriasis Area and Severity Index (PASI). r; Regression, Sig; Significant level
Discussion
In this study of the effect of micronutrients on the expression of Th1 and Th2 components, our data showed that clinical responses are associated with specific modulation of immune pathways. A shift to a Th2 pathway was based on greater GATA3 and IL-4 and fewer IFN-Υ, IL-12, and T-bet expression in the MT than in the NMT group.
These results supported previous studies on the role of MTX, in which pro-inflammatory cytokine expression decreased, and a shift from a Th1 to a Th2 pathway was reported (16, 17, 30).
We found greater GATA3 and IL-4 than IFN-Υ, IL-12, and T-bet expression in patients with PASI scores above 75. Therefore, we found the patients with improved clinical responses downregulates Th1 and upregulate Th2 components. In support of this finding, previous studies have shown higher IFN-Υ (5, 31) and lower IL-4 (31, 32) levels in sera of psoriatic patients than in healthy individuals, and correlated IFN-Υ levels with the psoriasis clinical severity score (5, 31). Furthermore, a decrease in serum IFN-Υ levels correlated with decreased PASI scores following methotrexate treatment (4). Regulation of the Th1/Th2 balance can be mediated by T-bet and GATA-3 (33). This research documented Th1 dominance in PBMCs of psoriatic patients in conjunction with significantly greater Tbet and less GATA-3 mRNA expression than in healthy subjects (26).
Overall, we found the mRNA cytokine expression followed similar trends even if they did not show statistically significant differences in clinical responses. Similar to our results, Xinqiang et al. (16) found no correlation between high expression of IL-4 due to MTX treatment in rheumatoid arthritis (RA) patients and clinical responses. These results could be due to either a need for more time to study this relationship in lymphocytes or a need for more of the selected cytokines or transcriptional repressors due to their probable cross regulation at the transcriptional level. We suggest further studies for a better understanding of the correlation between cytokine changes and clinical responses.
A potential association between micronutrient or dietary supplements and improvement of psoriasis lesions have not been studied. It is possible that micronutrients augment the immune response by improving T-lymphocyte function or one or more components of the innate immune system, or by blocking the activity of inflammatory cytokines in psoriasis patients. Retinoid regulate several elements of the immune response by suppressing IL-1, IFN-Υ, and TNF-α, the pro-inflammatory cytokines in psoriasis, and improving Th2 responses by enhancing IL-4, IL-5, and IL-10 secretion. The immune-modulatory role of 1,25-dihydroxyvitamin D3 (vitD3), with beneficial effects on Th1-mediated autoimmune diseases, was demonstrated through Th cell polarization by inhibiting production of the Th1 factors IFN-Υ and T-bet, and by augmenting Th2 cell development via IL-4, IL-5, IL-10, and GATA3 production (34). The clinical efficacy of vitD3 in psoriasis treatment was shown to be due to suppression of members of the IL-1 family, IL-12/23 p40, and TNF-α in psoriatic lesions (35), and in other Th1-associated diseases, including Crohn’s disease (10), through decreasing IL-2, TNF-α, and IFN-γ, and increasing IL-10 from Th2 immune cells. Vitamin E supplements improved indices of cell-mediated immunity and selenium supplements improved of T-cell function and control of the immune response in psoriasis lesions (36). Vitamin B12 aided in the maintenance of immune function and decreased pro-inflammatory cytokine expression (37), and dietary zinc, in activating NF-κB, induced expression of IL-1β; and TNF-α, and neutrophil infiltration during the early stages of cutaneous wound healing (38). Recently, Yousefzadeh et al found more significant decrement of IL-1β; and TNF-α levels followed with the better improvement of PASI score among psoriasis patients who were treated under micronutrients and MTX compare than who were treated with MTX only (39). Therefore, in the present study, the significant decrease in expression of Th1 pathway inflammatory cytokines and increase in expression of Th2 pathway cytokines in subjects receiving micronutrients plus MTX is likely due to the synergy between the two therapies, enhancing the clinical efficacy of MTX leading to this immunological pathway shift.
To our knowledge, this is the first study to combine MTX and micronutrients in psoriasis treatment and correlate clinical responses with the Th1/Th2 immunological pattern. Our findings indicate T cell modulation to the Th2 pathway and improved clinical responses are induced by MTX and MTX plus micronutrients. Other psoriasis treatments also modulate T cell responses in accordance with clinical responses, but we suggest micronutrients combined with MTX as the most cost-effective therapy in psoriasis patients. However, the small number of subjects in our study likely limits the data. The results should be confirmed by a larger study with more patients. We recommend conducting sensitive analyses like flow cytometry techniques for determining the involved subsets of monocytes in psoriasis patients under the respected therapy. Moreover, in vitro studies by cultured monolayer keratinocytes of skin biopsies from psoriasis patients could constitute a future step of the study to elucidate the cytokine effects on keratinocytes in order to assess the centrality of the related mediators in psoriasis pathogenesis.
Acknowledgements
The authors appreciate Dermatology Department of Ghaem Hospital, for their kind assistance in patients sampling. In addition, we kindly acknowledge the personnel of Immunology Research Center, Bu-Ali Research Institute for their help in Quantitative realtime PCR tests. This study was supported financially by Research Council of Mashhad University of Medical Sciences under research thesis code 910739.
The authors report that there is no conflict of interest. No financial support or other benefits from commercial sources were provided for this work.
References
- 1.Lowes MA, Lew W, Krueger JG. Current concepts in the immunopathogenesis of psoriasis. Dermatol Clin. 2004;22(4):349–69. doi: 10.1016/j.det.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Gisondi P, Girolomoni G. Biologic therapies in psoriasis: a new therapeutic approach. Autoimmun Rev. 2007;6(8):515–9. doi: 10.1016/j.autrev.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Nograles KE, Davidovici B, Krueger JG. New insights in the immunologic basis of psoriasis. Semin Cutan Med Surg. 2010;29(1):3–9. doi: 10.1016/j.sder.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pietrzak AT, Zalewska A, Chodorowska G, Krasowska D, Michalak-Stoma A, Nockowski P, et al. Cytokines and anticytokines in psoriasis. Clin Chim Acta. 2008;394(1-2):7–21. doi: 10.1016/j.cca.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Arican O, Aral M, Sasmaz S, Ciragil P. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;2005(5):273–9. doi: 10.1155/MI.2005.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghoreschi K, Mrowietz U, Rocken M. A molecule solves psoriasis? Systemic therapies for psoriasis inducing interleukin 4 and Th2 responses. J Mol Med (Berl) 2003;81(8):471–80. doi: 10.1007/s00109-003-0460-9. [DOI] [PubMed] [Google Scholar]
- 7.Jain S, Kaur IR, Das S, Bhattacharya SN, Singh A. T helper 1 to T helper 2 shift in cytokine expression: an autoregulatory process in superantigen-associated psoriasis progression? . J Med Microbiol. 2009;58(Pt 2):180–4. doi: 10.1099/jmm.0.003939-0. [DOI] [PubMed] [Google Scholar]
- 8.Wilson PB. Is dietary supplementation more common among adults with psoriasis? Results from the National Health and Nutrition Examination Survey. Complement Ther Med. 2014;22(1):159–65. doi: 10.1016/j.ctim.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Duarte G, Barbosa LO, Rosa M. The management of psoriasis through diet. Psoriasis: Targets and Therapy. 2012;2:45–53. [Google Scholar]
- 10.Ardizzone S, Cassinotti A, Trabattoni D, Manzionna G, Rainone V, Bevilacqua M, et al. Immunomodulatory effects of 1,25-dihydroxyvitamin D3 on TH1/TH2 cytokines in inflammatory bowel disease: an in vitro study. Int J Immunopathol Pharmacol. 2009;22(1):63–71. doi: 10.1177/039463200902200108. [DOI] [PubMed] [Google Scholar]
- 11.Ozkara S, Keles E, Ilhan N, Gungor H, Kaygusuz I, Alpay HC. The relationship between Th1/Th2 balance and 1alpha,25-dihydroxyvitamin D(3) in patients with nasal polyposis. Eur Arch Otorhinolaryngol. 2012;269(12):2519–24. doi: 10.1007/s00405-012-1967-x. [DOI] [PubMed] [Google Scholar]
- 12.Spilianakis CG, Lee GR, Flavell RA. Twisting the Th1/Th2 immune response via the retinoid X receptor: lessons from a genetic approach. Eur J Immunol. 2005;35(12):3400–4. doi: 10.1002/eji.200535588. [DOI] [PubMed] [Google Scholar]
- 13.Roenigk HH, Auerbach R, Maibach HI, Weinstein GD. Methotrexate in psoriasis: revised guidelines. J Am Acad Dermatol. 1988;19(1 Pt 1):145–56. doi: 10.1016/s0190-9622(88)80237-8. [DOI] [PubMed] [Google Scholar]
- 14.Herman S, Zurgil N, Deutsch M. Low dose methotrexate induces apoptosis with reactive oxygen species involvement in T lymphocytic cell lines to a greater extent than in monocytic lines. Inflamm Res. 2005;54(7):273–80. doi: 10.1007/s00011-005-1355-8. [DOI] [PubMed] [Google Scholar]
- 15.Alfageme Roldan F, Bermejo Hernando A, Calvo Gonzalez JL, Marques Sanchez P. [Cost Effectiveness of Treatments of Psoriasis with a PASI 75 and one Period of 12 Weeks] Rev Esp Salud Publica. 2015;90:E15. [PubMed] [Google Scholar]
- 16.Xinqiang S, Fei L, Nan L, Yuan L, Fang Y, Hong X, et al. Therapeutic efficacy of experimental rheumatoid arthritis with low-dose methotrexate by increasing partially CD4+CD25+ Treg cells and inducing Th1 to Th2 shift in both cells and cytokines. Biomed Pharmacother. 2010;64(7):463–71. doi: 10.1016/j.biopha.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Herman S, Zurgil N, Langevitz P, Ehrenfeld M, Deutsch M. Methotrexate selectively modulates TH1/TH2 balance in active rheumatoid arthritis patients. Clin Exp Rheumatol. 2008;26(2):317–23. [PubMed] [Google Scholar]
- 18.Farrar JD, Asnagli H, Murphy KM. T helper subset development: roles of instruction, selection, and transcription. J Clin Invest. 2002;109(4):431–5. doi: 10.1172/JCI15093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noble A. Review article: molecular signals and genetic reprogramming in peripheral T-cell differentiation. Immunology. 2000;101(3):289–99. doi: 10.1046/j.1365-2567.2000.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. Pillars article: A novel transcription factor, t-bet, directs th1 lineage commitment. Cell. 2000. 100: 655-669. J Immunol. 2015;194(7):2961–2975. [PubMed] [Google Scholar]
- 21.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH, et al. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295(5553):338–42. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 22.Mullen AC, High FA, Hutchins AS, Lee HW, Villarino AV, Livingston DM, et al. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001;292(5523):1907–10. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- 23.Lee HJ, Takemoto N, Kurata H, Kamogawa Y, Miyatake S, O'Garra A, et al. GATA-3 induces T helper cell type 2 (Th2) cytokine expression and chromatin remodeling in committed Th1 cells. J Exp Med. 2000;192(1):105–15. doi: 10.1084/jem.192.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawashima M, Miossec P. mRNA quantification of T-bet, GATA-3, IFN-gamma, and IL-4 shows a defective Th1 immune response in the peripheral blood from rheumatoid arthritis patients: link with disease activity. J Clin Immunol. 2005;25(3):209–14. doi: 10.1007/s10875-005-4092-4. [DOI] [PubMed] [Google Scholar]
- 25.Arakawa S, Hatano Y, Katagiri K. Differential expression of mRNA for Th1 and Th2 cytokine-associated transcription factors and suppressors of cytokine signalling in peripheral blood mononuclear cells of patients with atopic dermatitis. Clin Exp Immunol. 2004;135(3):505–10. doi: 10.1111/j.1365-2249.2004.02405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu K, Ye J, Wu M, Cheng H. Expression of Th1 and Th2 cytokine-associated transcription factors, T-bet and GATA-3, in peripheral blood mononuclear cells and skin lesions of patients with psoriasis vulgaris. Arch Dermatol Res. 2010;302(7):517–23. doi: 10.1007/s00403-010-1048-1. [DOI] [PubMed] [Google Scholar]
- 27.Montaudie H, Sbidian E, Paul C, Maza A, Gallini A, Aractingi S. Methotrexate in psoriasis: a systematic review of treatment modalities, incidence, risk factors and monitoring of liver toxicity. J Eur Acad Dermatol Venereol. 2011;25 Suppl 2:12–8. doi: 10.1111/j.1468-3083.2011.03991.x. [DOI] [PubMed] [Google Scholar]
- 28.Marks R, Barton SP, Shuttleworth D, Finlay AY. Assessment of disease progress in psoriasis. Arch Dermatol. 1989;125(2):235–40. [PubMed] [Google Scholar]
- 29.Czarnecka-Operacz M, Sadowska-Przytocka A. The possibilities and principles of methotrexate treatment of psoriasis - the updated knowledge. Postepy Dermatol Alergol. 2014;31(6):392–400. doi: 10.5114/pdia.2014.47121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thirupathi A, Elango T, Subramanian S, Gnanaraj P. Methotrexate regulates Th-1 response by suppressing caspase-1 and cytokines in psoriasis patients. Clin Chim Acta. 2016;453:164–9. doi: 10.1016/j.cca.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 31.Szegedi A, Aleksza M, Gonda A, Irinyi B, Sipka S, Hunyadi J, et al. Elevated rate of Thelper1 (T(H)1) lymphocytes and serum IFN-gamma levels in psoriatic patients. Immunol Lett. 2003;86(3):277–80. doi: 10.1016/s0165-2478(03)00025-7. [DOI] [PubMed] [Google Scholar]
- 32.Schlaak JF, Buslau M, Jochum W, Hermann E, Girndt M, Gallati H, et al. T cells involved in psoriasis vulgaris belong to the Th1 subset. J Invest Dermatol. 1994;102(2):145–9. doi: 10.1111/1523-1747.ep12371752. [DOI] [PubMed] [Google Scholar]
- 33.Park HJ, Lee CM, Jung ID, Lee JS, Jeong YI, Chang JH, et al. Quercetin regulates Th1/Th2 balance in a murine model of asthma. Int Immunopharmacol. 2009;9(3):261–7. doi: 10.1016/j.intimp.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 34.Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O'Garra A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167(9):4974–80. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 35.Balato A, Schiattarella M, Lembo S, Mattii M, Prevete N, Balato N, et al. Interleukin-1 family members are enhanced in psoriasis and suppressed by vitamin D and retinoic acid. Arch Dermatol Res. 2013;305(3):255–62. doi: 10.1007/s00403-013-1327-8. [DOI] [PubMed] [Google Scholar]
- 36.Naziroglu M, Yildiz K, Tamturk B, Erturan I, Flores-Arce M. Selenium and psoriasis. Biol Trace Elem Res. 2012;150(1-3):3–9. doi: 10.1007/s12011-012-9479-5. [DOI] [PubMed] [Google Scholar]
- 37.Al-Daghri NM, Rahman S, Sabico S, Yakout S, Wani K, Al-Attas OS, et al. Association of Vitamin B12 with Pro-Inflammatory Cytokines and Biochemical Markers Related to Cardiometabolic Risk in Saudi Subjects. Nutrients. 2016;8(9) doi: 10.3390/nu8090460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim Y, Levy M, Bray TM. Dietary zinc alters early inflammatory responses during cutaneous wound healing in weanling CD-1 mice. J Nutr. 2004;134(4):811–6. doi: 10.1093/jn/134.4.811. [DOI] [PubMed] [Google Scholar]
- 39.Yousefzadeh H, Jabbari Azad F, Banihashemi M, Rastin M, Mahmoudi M. Evaluation of psoriasis severity and inflammatory responses under concomitant treatment with methotrexate plus micronutrients for psoriasis vulgaris: a randomized double blind trial. Acta Dermatovenerol Alp Pannonica Adriat. 2017;26(1):3–9. doi: 10.15570/actaapa.2017.2. [DOI] [PubMed] [Google Scholar]


