Abstract
Background:
Dyslipidemia is considered an independent risk factor for coronary heart disease (CHD). In the present study, we examined lipid profiles and paraoxonase 1 (PON1) activity and atherogenic indexes status and the relationship of PON1 activity by high-density lipoprotein (HDL) and atherogenic indexes in CHD patients and healthy people.
Methods:
The aim of the study was to compare PON1, lipid profiles, and atherogenic indexes in CHD patients and healthy people as controls. This study enrolled 50 CHD patients and 50 matched healthy controls. Serum activities of PON1 and levels of triglyceride (TG), total cholesterol (TC), low density lipoprotein (LDL), very low density lipoprotein (VLDL), high-density lipoprotein cholesterol (HDL-C), fasting blood glucose (FBG), and atherogenic indexes were analyzed. Data were analyzed by unpaired Student’s t tests. Coefficients of correlation were calculated using Pearson’s correlation analysis.
Results:
Levels of TG, TC, LDL, VLDL, FBG, and atherogenic indexes, atherogenic coefficients, and cardiac risk ratios were significantly greater in CHD patients than in controls. Paraoxonase 1 activity and HDL-C levels were significantly less in CHD patients than in controls. Also, PON1 activity correlated positively with HDLC and negatively with atherogenic coefficient, and cardiac risk ratios 1 and 2 in CHD patients.
Conclusion:
This study showed that CHD is associated with high lipid levels and atherogenic indexes, and low PON1 activity and HDL-C concentrations. Coronary heart disease is a pernicious disease requiring prolonged medical management and hypolipidemic drugs.
Key Words: Atherogenic index, Coronary heart disease, Lipid profile, Paraoxonase 1 activity
Introduction
The prevalence of coronary heart disease (CHD) worldwide is rapidly rising (1). CHD is one of the main causes of death in Iran (2). It is widely accepted that CHD is associated with hypertension and elevated blood levels of low-density lipoprotein (LDL), total cholesterol (TC), and triglycerides (TG) (3, 4). In contrast, a low level of high-density lipoprotein (HDL) is a risk factor for mortality from CHD (5). Abnormalities of lipoproteins are responsible for increased incidence of microvascular and macrovascular complications in CHD (1).
Dyslipidemia (high TG, high TC, and low HDL) is common in patients with CHD. Also, a TC to highdensity lipoprotein cholesterol (HDL-C) ratio > 4.5 is considered the most powerful predictor of CHD (6). Dyslipidemia is a risk factor for increased CHD incidence and increases the mortality rate in CHD (7). Monitoring and managing lipid profiles and using hypolipidemic drugs reduce CHD complications and associated mortality.
Human serum paraoxonase 1 (PON1) is an HDL-C-bound enzyme considered to be the major Downloaded from rbmb.net at 14:21 +0430 on Friday July 21st 2017 Rep. Biochem. Mol. 2 Biol, Vol.6, No.1, Oct 2017 determinant of the antioxidant action of HDL-C (8). PON1 has detoxification activity in atherosclerotic processes (9). Several studies reported that PON1 and HDL protect against LDL oxidative modification (10). Other studies showed that PON1 can prevent lipid peroxide accumulation on LDL in vitro and in vivo (9). Also, serum PON1 activity has been shown to be reduced in familial hypercholesterolemia and diseases associated with accelerated atherogenesis (11). Paraoxonase 1’s antiatherogenic activities protect lipoprotein particles from free radical oxidation, hydrolyze oxidized cholesteryl esters and phosphatidylcholine core aldehydes, and degrade hydrogen peroxide (11, 12). Monitoring the trends in cardiovascular complications via PON1 activity is critical for management of patients with CHD (12).
Therefore, we hypothesized that serum PON1 activity and HDL levels would be decreased, and cholesterol, TGs, and atherogenic indexes would be increased, in CHD. Few studies have evaluated atherogenic indexes status and correlated PON1 activity with HDL and atherogenic indexes in CHD. The objectives of this study were to evaluate PON1 activity, lipid profiles, and atherogenic indexes status and correlate PON1 activity with HDL and atherogenic indexes in CHD.
Materials and Methods
Patient selection
This study was conducted on 50 CHD patients and 50 healthy controls at Shahid Madani Hospital, Khorramabad University of Medical Sciences, Khorramabad, Iran. The study was approved by the Institutional Ethics Committee. Written informed consent was obtained from the patients in the local language. This is an observational and cross-sectional study. The criteria for selection of cases were:
Inclusion criteria
I. The subjects were 35-65 years of age.
II. Coronary heart disease patients were diagnosed by coronary angiography.
Exclusion criteria:
I. Diabetic patients
II. Patients with any concurrent illness such as chronic liver disease and hypothyroidism.
IV. Patients receiving medications including as diuretics, steroids, oral contraceptives, and beta blockers
Collection of blood samples
After an overnight fast of 10-12 hours, 5 ml of whole blood was collected via vena puncture between 7:00 and 8.00 am.
Determination of PON1 activity
Paraoxonase 1 activity was determined using paraoxon as the substrate and measured by increases in the absorbance at 412 nm due to the formation of 4- nitrophenol as previously described (8). The activity was measured at 25 °C by adding 50 µl of serum to 1 ml of Tris-HCl buffer (100 mM at pH 8.0) containing 2 mM CaCl2 and 5 mM paraoxon. The rate of generation of 4- nitrophenol was determined at 412 nm. Enzymatic activity was calculated using a molar extinction coefficient of 17,100 M-1 cm-1 (8).
Determination of lipid profile and atherogenic indexes
The serum levels of triglyceride (TG), total cholesterol (TC), low density lipoprotein (LDL), very low density lipoprotein (VLDL), fasting blood glucose, (FBG), and HDL-C, and the atherogenic indexes of all the subjects were analyzed. Total cholesterol and TG concentrations were measured by biochemical analyses using commercial kits (Olympus AU-600, Tokyo, Japan). HDL-C was analyzed with a Pars Azmoon kit from Iran. LDL and VLDL were determined using the Friedewald et al. equation (13). The atherogenic index ((units) (log (TG/HDL-C)), atherogenic coefficient (TCHDL- C)/HDL-C), Cardiac Risk Ratio 1: (TC/HDL-C) and Cardiac Risk Ratio 2: (LDL/HDL-C) were determined using the Ikewuchi and Ikewuchi equation (14-16).
Statistical analyses
Data were analyzed using unpaired Student’s t tests. Coefficients of correlation were calculated using Pearson’s correlation analysis. Statistical analyses were performed using the software package for statistical analysis version 13 (SPSS) for Windows software. A P value of <0.05 was considered statistically significant.
Results
Fasting blood glucose, TG, TC, LDL, and VLDL concentrations were significantly greater in CHD patients than in controls, while PON1 activity and HDL concentrations were significantly less in CDH patients than in controls (Table 1).
Table 1.
Lipid Profile and PON1 activity in coronary heart disease (CHD)
| Parameter | Control | CHD | P Value |
|---|---|---|---|
| FBG (mg/dl) | 90.90± 16.25 | 105.3± 19.35 | 0.005 |
| PON1 Unit/min | 9.42± 7.18 | 4.14± 2.11 | 0.0001 |
| TG (mg/dl) | 114.73±31.63 | 195.21± 102.59 | 0.001 |
| TC (mg/dl) | 126.46±18.68 | 166.33±30.66 | 0.001 |
| HDL-C (mg/dl) | 48.08 ± 9.69 | 40.75± 5.49 | 0.0001 |
| LDL (mg/dl) | 55.43± 23.14 | 86.54± 33.04 | 0.001 |
| VLDL (mg/dl) | 22.94±6.33 | 39.04±20.54 | 0.001 |
Values are represented as Means ± SDs
The atherogenic index, atherogenic coefficient, and Cardiac Risk Ratios 1 and 2 were significantly greater in CHD patients than in controls (Table 2).
Table 2.
Atherogenic index, atherogenic coefficient, and cardiac risk ratio in coronary heart disease (CHD)
| Parameter | Control | CHD | P Value |
|---|---|---|---|
| Atherogenic index ((units) (log (TG/HDL-C)) (TG/HDL-C)) | 0.37±0.16 | 0.64±0.17 | 0.001 |
| Atherogenic Coefficient (TC-HDL-C)/HDLC) | 1.76±0.76 | 3.16±0.95 | 0.001 |
| Cardiac Risk Ratio 1 (TC/HDL-C) | 2.76 ± 0.76 | 4.16 ± 0.94 | 0.001 |
| Cardiac Risk Ratio 2 (LDL/HDL-C) | 1.26 ± 0.67 | 2.19± 0.98 | 0.001 |
Values are represented as Means ± SDs
Paraoxonase 1 activity correlated positively with HDL ((r = 0.261, p = 0.045, Fig. 1) and negatively with atherogenic coefficient (r = -0.083, p = 0.303, Fig. 2), cardiac risk ratio 1 (r = -0.071, p = 0.33, Fig. 3) and cardiac risk ratio 2 (r = -0.45, p = 0.39, Fig. 4).
Fig. 1.
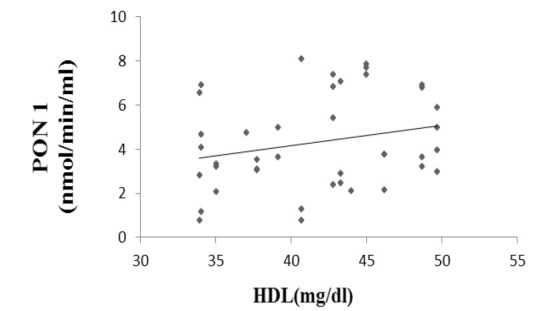
Correlation between serum PON1 activity and HDL-C levels. (r = 0.261, p = 0.045).
Fig. 2.
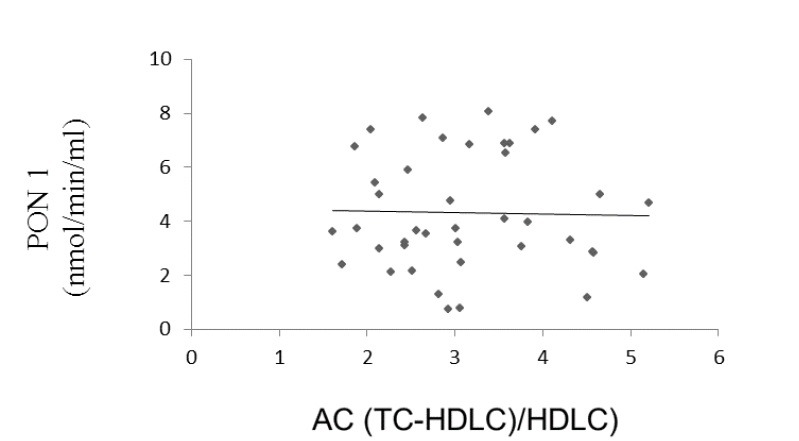
Correlation between serum PON1 activity and AC levels (Atherogenic Coefficient (TC-HDLC)/HDL-C)). (r = -0.083, p = 0.303).
Fig. 3.
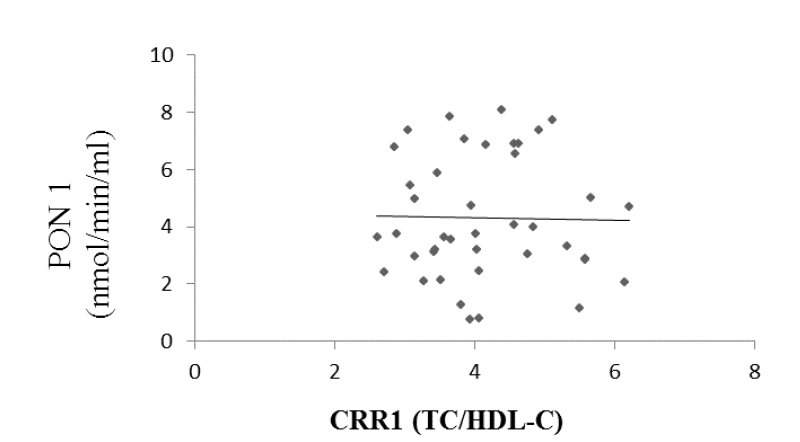
Correlation between serum PON1 activity and CRR1 levels (Cardiac Risk Ratio 1 (TC/HDL-C)). (r = -0.071, p = 0.33).
Fig. 4.
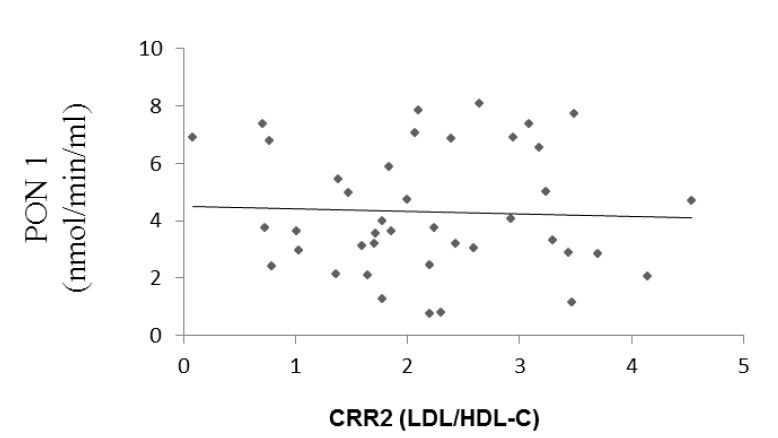
Correlation between serum PON1 activity and CRR2 levels (Cardiac Risk Ratio 2 (LDL/HDL-C)). (r = -0.045, p = 0.39).
Discussion
Serum lipid profile and atherogenic index
The most relevant findings of this study were significant increases in serum levels of FBG, TG, TC, VLDL, LDL, atherogenic index, atherogenic coefficient, and cardiac risk ratios 1 and 2 in DHC. Studies have shown that total cholesterol, triglycerides, LDL concentrations, and LDL/HDL ratios are significantly greater in CHD patients than in healthy people (17). Many studies have shown that total cholesterol and LDL are major risk factors for atherosclerotic vascular diseases. Also, epidemiological studies have shown a correlation between LDL and CHD (18). It has also been demonstrated that lipid-lowering drugs, such as statins, reduce the risk of cardiovascular diseases (17). It has also been shown that HDL-C concentration is inversely related to the risk of CHD (19). Atherogenic indices, atherogenic coefficient, and cardiac risk ratio are powerful indicators of the risk of heart disease: the higher the value, the higher the risk of developing CHD and vice versa (20). Many studies have shown that atherogenic indices, atherogenic coefficient, and cardiac risk ratios are major risk factors for atherosclerotic vascular disease and its complications. The results of our study agree with others researchers’ studies showing that total cholesterol, triglycerides, and LDL concentrations, and atherogenic indices, atherogenic coefficients and cardiac risk ratios are greater in CHD patients than in healthy people. Therefore, reduction of LDL cholesterol and atherogenic indices have been considered to reduce risk of CHD.
Serum activity of PON1 and correlation with HDL and atherogenic index in CHD
The most relevant finding of this study was a significant decrease in serum PON1 activity in the CHD patients compared with healthy controls. Also, PON1 activity correlated positively (r = 0.261, p = 0.045) with HDL in CHD. Paraoxonase 1 is an antioxidant that inhibits oxidative modification of LDL and contributes most of the antioxidative activity that has been attributed to HDL (21, 22). Paraoxonase 1 activity was positively correlated with HDL-C levels (23) and inversely correlated with atherogenic index, suggesting that decreased PON1 activity may be, in part, due to consumption of PON1 for the prevention of oxidation (8). This strongly suggests that decreased PON1 activity may be at least partially related to the consumption of PON1 caused by oxidative stress process (24). Recently, roles for PON1 in a number of processes have been studied, including lipid and lipoprotein metabolism, as well as for their antiatherogenic and antioxidant properties (8, 25). This study showed strong evidence of CHD associated with low activity of PON1 and high lipid levels and atherogenic indexes.
The association between PON activity and the development of atherosclerosis has been shown in animal and human studies (26). HDL isolated from normal animals protected LDL from oxidation while HDL isolated from PON-deficient animals did not. Studies have shown that PON deficiency developed atherosclerotic lesions but animals with overexpressed PON1 were resistant to atherosclerosis (27). Also, epidemiological studies have revealed an association between decreased PON1 levels and an increased risk for atherosclerosis (28).
Our results agreed with those of earlier’ studies showing increased PON1 activity was in CHD and positively correlated with HDL. The mechanism behind this association is unknown. It is possible that the pathogenesis of decreased HDL may also play a role in the reduction of PON1 activity (29).
In conclusion, we found significantly lower levels of HDL-C and PON1 activity and significantly higher levels of FBG, TG, TC, VLDL, LDL, atherogenic index, atherogenic coefficient and cardiac risk ratios in CHD. These findings support the hypothesis that decreased PON1 activity is associated with the decreased HDL concentration found in CHD. Our findings suggest that decreased PON1 activity and HDL concentration and increased FBG, TG, TC, VLDL, and LDL concentrations, atherogenic index, atherogenic coefficient, and cardiac risk ratios may be involved in the early pathogenesis of atherosclerotic heart disease in CHD. However, long-term clinical studies are needed to clarify the pathophysiological role of serum PON1 activity and lipid profile, and correlation of PON1 activity with HDL and atherogenic index in CHD.
Acknowledgements
The authors thank the officers in charge of the Lorestan University of Medical Sciences for providing a grant that supported this study. In addition, the authors thank all patients that participated in this study. The authors declare that they have no conflicts of interest.
References
- 1.Yatsuya H, Li Y, Hilawe EH, Ota A, Wang C, Chiang C, Zhang Y, et al. Global trend in overweight and obesity and its association with cardiovascular diseaseincidence. Circ J. 2014;78(12):2806–18. doi: 10.1253/circj.cj-14-0850. [DOI] [PubMed] [Google Scholar]
- 2.Abdolrahimi S, Sanati H, Fatahian A. Evaluation of Percutaneous Coronary Intervention and stenting of Left Main Coronary Artery Stenosis in Tehran's Rajaie and Lavasani Hospitals from 2010 to 2011. Res Cardiovasc Med. 2013;2(4):181–4. doi: 10.5812/cardiovascmed.12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rad EM, Assadi F. Management of hypertension in children with cardiovascular disease and heart failure. Int J Prev Med. 2014;5(Suppl 1):S10–6. [PMC free article] [PubMed] [Google Scholar]
- 4.Agouridis AP, Rizos CV, Elisaf MS, Filippatos TD. Does combination therapy with statins and fibrates prevent cardiovascular disease in diabetic patients with atherogenic mixed dyslipidemia? Rev Diabet Stud. 2013;10(2-3):171–90. doi: 10.1900/RDS.2013.10.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agouridis AP, Rizos CV, Elisaf MS, Filippatos TD. Does combination therapy with statins and fibrates prevent cardiovascular disease in diabetic patients with atherogenic mixed dyslipidemia? Rev Diabet Stud. 2013;10(2-3):171–90. doi: 10.1900/RDS.2013.10.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tietge UJ. Hyperlipidemia and cardiovascular disease: inflammation, dyslipidemia, and atherosclerosis. Curr Opin Lipidol. 2014;25(1):94–5. doi: 10.1097/MOL.0000000000000051. [DOI] [PubMed] [Google Scholar]
- 7.Ferrières J, Amber V, Crisan O, Chazelle F, Jünger C, Wood D. Total lipid management and cardiovascular disease in the dyslipidemia international study. Cardiology. 2013;125(3):154–63. doi: 10.1159/000348859. [DOI] [PubMed] [Google Scholar]
- 8.Ahmadvand H, Ghasemi Dehnoo M, Dehghani A, Bagheri S, Cheraghi RA. Serum paraoxonase 1 status and its association with atherogenic indexes in gentamicin-induced nephrotoxicity in rats treated with coenzyme Q10. Ren Fail. 2014;36(3):413–8. doi: 10.3109/0886022X.2013.865154. [DOI] [PubMed] [Google Scholar]
- 9.Wang F, Liu Y, Cong Y, Li P, Li Y, Yan Z, et al. Shift of the interconnection from the reaction system of paraoxonase 1 to the peroxidation reaction system of myeloperoxidase with HDL-C levels: A marker of atherosclerosis in patients with normal cholesterol levels. Clin Chim Acta. 2015;438:370–5. doi: 10.1016/j.cca.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Deakin SP, Bioletto S, Bochaton-Piallat ML, James RW. HDL associated paraoxonase-1 can redistribute to cell membranes and influence sensitivity to oxidative stress. Free Radic Biol Med. 2011;50(1):102–9. doi: 10.1016/j.freeradbiomed.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Van Himbergen TM, van Tits LJ, Hectors MP, de Graaf J, Roest M, Stalenhoef AF. Paraoxonase-1 and linoleic acid oxidation in familial hypercholesterolemia. Biochem Biophys Res Commun. 2005;333(3):787–93. doi: 10.1016/j.bbrc.2005.05.176. [DOI] [PubMed] [Google Scholar]
- 12.Wang M, Lang X, Cui S, Zou L, Cao J, Wang S, et al. Quantitative assessment of the influence of paraoxonase 1 activity and coronary heart disease risk. DNA Cell Biol. 2012;31(6):975–82. doi: 10.1089/dna.2011.1478. [DOI] [PubMed] [Google Scholar]
- 13.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of LDL-C in plasma without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 14.Ikewuchi JC, Ikewuchi JC. Alteration of plasma lipid profiles and atherogenic indeces by Stachytarpheta iamaicensis L. (Vahl) Biokemistri. 2009;21(2):71–7. [Google Scholar]
- 15.Ahmadvand H, Ghasemi-Dehnoo M. Antiatherogenic, hepatoprotective, and hypolipidemic effects of coenzyme Q10 in alloxan-induced type 1 diabetic rats. ARYA Atheroscler. 2014 Jul;10(4):192–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmadvand H, Noori A, Ghasemi Dehnoo M, Bagheri S, Cheraghi RA. Hypoglycemic, hypolipidemic and antiatherogenic effects of oleuropein in alloxan-induced Type 1 diabetic rats. Asian Pac J Trop Dis. 2014;4(Suppl 1):S421–S425. [Google Scholar]
- 17.Gamboa CM, Safford MM, Levitan EB, Mann DM, Yun H, Glasser SP, et al. Statin underuse and low prevalence of LDL-C control among U.S. adults at high risk of coronary heart disease. Am J Med Sci. 2014;348(2):108–14. doi: 10.1097/MAJ.0000000000000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benge CD, Markley B, McFarland MS. Role of aggressive LDL reduction in patients with coronary heart disease. South Med J. 2012;105(1):48–55. doi: 10.1097/SMJ.0b013e31823c4242. [DOI] [PubMed] [Google Scholar]
- 19.Landmesser U. Coronary artery disease: HDL and coronary heart disease--novel insights. Nat Rev Cardiol. 2014;11(10):559–60. doi: 10.1038/nrcardio.2014.128. [DOI] [PubMed] [Google Scholar]
- 20.Baliarsingh S, Sharma N, Mukherjee R. Serum uric acid: marker for atherosclerosis as it is positively associated with "atherogenic index of plasma". Arch Physiol Biochem. 2013;119(1):27–31. doi: 10.3109/13813455.2012.732580. [DOI] [PubMed] [Google Scholar]
- 21.Kota SK, Meher LK, Kota SK, Jammula S, Krishna SV, Modi KD. Implications of serum paraoxonase activity in obesity, diabetes mellitus, and dyslipidemia. Indian J Endocrinol Metab. 2013;17(3):402–12. doi: 10.4103/2230-8210.111618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Razavi AE, Ani M, Pourfarzam M, Naderi GA. Associations between high density lipoprotein mean particle size and serum paraoxonase-1 activity. J Res Med Sci. 2012;17(11):1020–6. [PMC free article] [PubMed] [Google Scholar]
- 23.Dullaart RP, Otvos JD, James RW. Serum paraoxonase-1 activity is more closely related to HDL particle concentration and large HDL particles than to HDL cholesterol in Type 2 diabetic and non-diabetic subjects. Clin Biochem. 2014;47(12):1022–7. doi: 10.1016/j.clinbiochem.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Eren E, Abuhandan M, Solmaz A. Taşkın. Serum paraoxonase/arylesterase activity and oxidative stress status in children with metabolic syndrome. J Clin Res Pediatr Endocrinol. 2014;6(3):163–8. doi: 10.4274/jcrpe.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghaffari T, Nouri M, Irannejad E, Rashidi MR. Effect of vitamin e and selenium supplement on paraoxonase-1 activity, oxidized low density lipoprotein and antioxidant defense in diabetic rats. Bioimpacts. 2011;1(2):121–8. doi: 10.5681/bi.2011.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding J, Chen Q, Zhuang X, Feng Z, Xu L, Chen F. Low Paraoxonase 1 Arylesterase Activity and High von Willebrand Factor Levels are Associated with Severe Coronary Atherosclerosis in Patients with Non-Diabetic Stable Coronary Artery Disease. Med Sci Monit. 2014;20:2421–9. doi: 10.12659/MSM.890911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng CJ, Bourquard N, Grijalva V, Hama S, Shih DM, Navab M, et al. Paraoxonase-2 deficiency aggravates atherosclerosis in mice despite lower apolipoprotein-B-containing lipoproteins: anti-atherogenic role for paraoxonase-2. J Biol Chem. 2006;281(40):29491–500. doi: 10.1074/jbc.M605379200. [DOI] [PubMed] [Google Scholar]
- 28.Gungor O, Kismali E, Sisman AR, Kircelli F, Asci G, Topal K, et al. The associations between serum paraoxonase 1 activity and carotidatherosclerosis in renal transplant patients. Clin Nephrol. 2013 Sep;80(3):198–202. doi: 10.5414/CN107855. [DOI] [PubMed] [Google Scholar]
- 29.Siewert S, Gonzalez II, Lucero RO, Ojeda MS. Association of cholesteryl ester transfer protein genotypes with paraoxonase-1 activity, lipid profile and oxidative stress in type 2 diabetes mellitus: A study in San Luis, Argentina. J Diabetes Investig. 2015;6(1):67–77. doi: 10.1111/jdi.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]


