Abstract
Objective
To systematically evaluate the prognosis in patients with breast cancer with ipsilateral supraclavicular lymph node metastasis (SLNM) versus patients with stage IIIb/c or IV breast cancer, so as to provide evidence for clinical practice and research.
Methods
Computer retrieval from PubMed, Cochrane Libratory, CNKI (China National Knowledge Infrastructure), CBM and Wanfang Database with the assistance of other retrieval tools. All the studies evaluating the prognosis in patients with breast cancer with ipsilateral supraclavicular lymph node metastasis versus patients with stage IIIb/c or IV breast cancer were collected. Quality assessment was performed for the included data based on the quality assessment criteria appropriate for this study. Meta-analysis was performed using RevMan 5.3 software.
Results
A total of four references (1277 patients) were included. Assessment of influences on prognosis: As compared to the stage IIIb/c group, the 5-year survival rate was slightly lower in the SLNM group (relative risk (RR) 0.79; 95% confidence interval (CI) 0.59–1.06; Z = 1.55, P = 0.12), but there was no statistical significance; in contrast, the 5-year survival rate was significantly increased in the SLNM group as compared to the stage IV group (RR = 2.70; 95%CI: 1.36–5.37; Z = 2.84, P = 0.005). As compared to the stage IIIb/c group, the 5-year disease-free survival rate was lower in the SLNM group (RR = 0.65; 95%CI: 0.40–1.05; Z = 1.75, P = 0.08); however, there was no statistical significance.
Conclusions
In patients with advanced breast cancer receiving combined therapy, the prognosis in patients with breast cancer with ipsilateral SLNM was significantly better than in those with stage IV breast cancer, and slightly worse than those with stage IIIb/c breast cancer. However, with the scarcity and poor quality of these observational studies, the long-term prognosis remains to be further verified in large-sample, high-quality studies.
Keywords: Breast cancer, Supraclavicular lymph node metastasis, Ipsilateral, Meta-analysis
The incidence of breast cancer with ipsilateral supraclavicular lymph node metastasis (SLNM) without distant metastasis is as low as 1–4%.1 In the 5th edition of the AJCC-TNM breast cancer staging system, the stage assigned to breast cancer with SLNM was modified from N3 to M1, possibly because ipsilateral SLNM in breast cancer is typically a sign of a poor prognosis as the majority of the patients would develop distant metastasis within one year.2, 3, 4 In the early 21st century, Brito et al5 reported for the first time a significantly better prognosis in patients with breast cancer with ipsilateral SLNM than in those with distant metastasis after receiving combined therapy including surgery, radiotherapy and chemotherapy. In the 6th edition of the AJCC-TNM breast cancer staging system, breast cancer with ipsilateral SLNM, which was no longer regarded as distant metastasis, was re-classified as stage IIIc instead of stage IV.6, 7 Nevertheless, such a staging has not been adequately evidenced, and no consensus has yet been reached on the selection of the treatment regimen for breast cancer with ipsilateral SLNM. Through meta-analysis of the literature on breast cancer with ipsilateral SLNM, this study was intended to explore its clinical relevance, in an attempt to provide references for further clinical practice and research.
Methods
Our meta-analysis was conducted in accordance with the ‘Preferred Reporting Items for Systematic Reviews and Meta-analysis’ (PRISMA) guidelines.8
Reference search strategies
Computer retrieval for the references published from 1 January, 2000 to 21 December, 2015 was performed from Pubmed, Cochrane Library, CNKI (China National Knowledge Infrastructure), CBM and Wanfang Data. All the studies comparing the prognosis in patients with breast cancer with ipsilateral SLNM vs. stage IIIb/c or IV breast cancer were collected by manual tracing or internet searching at Google Academics. Search items including Medical Subject Headings (MeSH) words and text words were related to breast cancer, ipsilateral supraclavicular lymph node metastasis, outcome and prognosis. References published during the years 2000–2015 and written simply in English were to be collected. To indentify more studies, we manually searched the reference lists of selected articles or review articles. We also contacted authors for additional data if necessary.
Inclusion criteria
Studies that satisfied the following criteria were included in this meta-analysis: 1) prospective or retrospective studies with the follow-up duration more than five years; 2) breast cancer patients with ipsilateral SLNM without distant metastasis and patients with stage IIIb/c or IV breast cancer as evidenced by imaging or pathological diagnosis; 3) reference must provide 5-year overall survival (OS) rate or 5-year disease-free survival (DFS) rate between two groups; 4) reference must have a sample size of at least 30 patients in each group. References published on the same population were to be reduced so that only the study with the best quality or the largest sample size was to be included in the study.
Data extraction
The references were selected by two reviewers (Xu-Hong Liu, Lei Zhang) independently according to the pre-defined inclusion criteria. Discrepancies were resolved through discussion with Bo Chen. The following data were extracted in each study, including the first author's name, the year of publication, country, the follow-up duration, method of outcome assessment, the diagnosis measurement of breast cancer, whether combined therapy was used or not, and the sample size.
Reference quality assessment
Our meta-analysis used the Newcastle–Ottawa Quality Assessment Scale (NOS) to evaluate the quality of each included study.9 The studies were assessed in three areas: the selection of exposed and unexposed participants; the comparability of the groups; and the assessment of the outcome. Total scores of each study range from one to nine; with nine being the maximum and ≥7 scores was considered high quality.
Statistical analyses
An I2 test was used for evaluating heterogenicity between different studies' results. When statistical homogenicity was found between different studies (I2 < 50%), a fixed-effect model was used for analysis; a random-effect model was used when statistical heterogenicity was found between different studies (I2 ≥ 50%).10 Potential publication bias was evaluated by a funnel plot. All analyses were performed with RevMan 5.3 software.
Results
Reference retrieval results
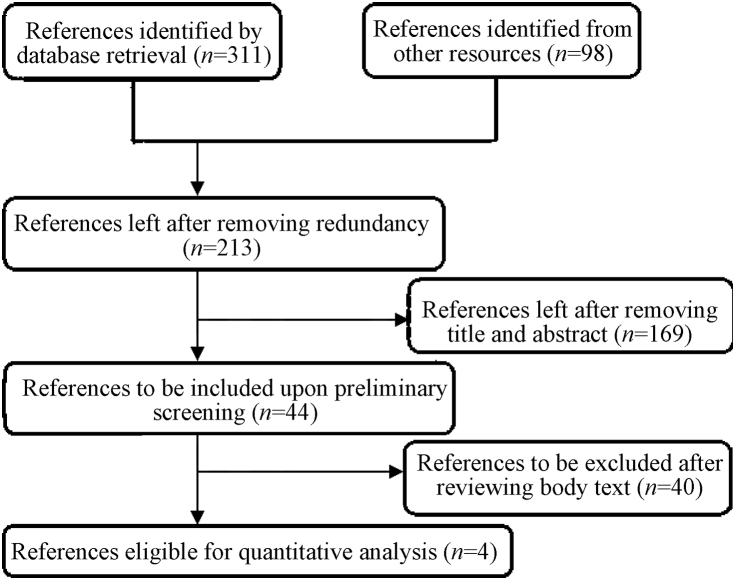
Preliminary retrieval identified 311 relevant references, of which 98 were excluded by Endnote Software due to redundancy and 169 were excluded by reviewing the title and abstract, leaving 44 preliminarily included. Among them 40/44 were excluded after reviewing the body text, leaving four references finally included that reported a total of 1277 patients (Fig. 1).
Fig. 1.
Flow diagram of reference selection procedures.
General characteristics and methodology evaluation of the included studies
The four studies finally included are comparable; as none of them report statistically significant differences between the two groups in clinical stage, treatment approach, or follow-up duration, etc., suggesting general consistency between groups. The general characteristics of the included references are presented in Table 1, and the methodology quality assessment is presented in Table 2.
Table 1.
General characteristics of included studies.
| Author | Year | Country | Follow-up duration | Outcome assessment | Diagnosis measurement | Combined therapy | Sample size |
5-year OS rate |
5-year DFS rate |
Quality score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SLNM | Ⅲb/c | Ⅳ | SLNM | Ⅲb/c | Ⅳ | SLNM | Ⅲb/c | Ⅳ | ||||||||
| Chen et al11 | 2006 | China | 58.3 months | Medical records | Serum tumor marker/X-ray/bonescan/ultrasound scan/computed tomographic scan | Yes | 63 | 151 | 599 | 33.6% | 34.9% | 9.1% | _ | _ | _ | 7 |
| Ogino et al12 | 2011 | Japan | 42.1 months | Breast cancer database | Pathologic proof | Unknown | 32 | 152 | 63 | 34.4% | 48.4% | 19.8% | 25.0% | 38.6% | _ | 7 |
| Dellapasqua et al13 | 2014 | Italy | 84.0 months | Institutional database | Lymph node biopsy/positron emission tomography/lymph node ultrasonography | Yes | 42 | 65 | _ | 57.1% | 96.9% | _ | 38.8% | 84% | _ | 8 |
| Noh et al14 | 2015 | Korea | 57.4 months | Medical records | Mammography/breast ultrasonography/MRI/PET/cytologically confirmed | Yes | 81 | 29 | _ | 75.3% | 77.2% | _ | 58.7% | 65.2% | _ | 7 |
Table 2.
Quality assessment of included references.
Results of meta-analysis
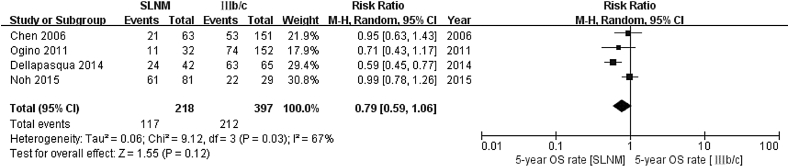
Five-year overall survival rate (SLNM vs IIIb/c) As heterogenicity was not shown between the four references included (χ2 = 9.12, P = 0.03, I2 = 67%), a random-effect model was used. As compared to the stage IIIb/c group, the 5-year survival rate was lower in the SLNM group (relative risk (RR) = 0.79; 95% confidence interval (CI): 0.59–1.06; Z = 1.55, P = 0.12) (Fig. 2).
Fig. 2.
Meta analysis results of 5-year survival rate in SLNM group vs. stage IIIb/c breast cancer group.
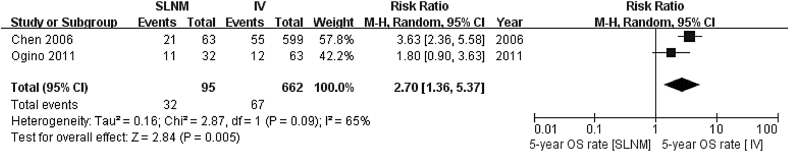
Five-year overall survival rate (SLNM vs. IV) As heterogenicity was shown between two included references (χ2 = 2.87, P = 0.09, I2 = 65%), a random-effect model was used. The 5-year survival rate significantly increased in the SLNM group as compared to the stage IV group (RR = 2.70; 95%CI: 1.36–5.37; Z = 2.84, P = 0.005), as illustrated in Fig. 3.
Fig. 3.
Meta analysis results of 5-year survival rate in SLNM group vs. stage IV breast cancer group.
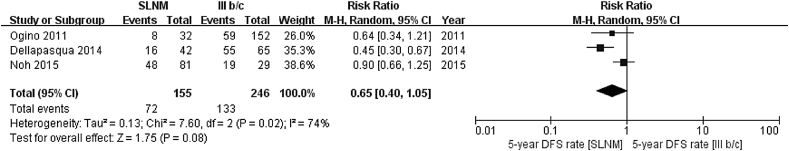
Five-year disease-free survival rate (SLNM vs. IIIb/c) As heterogenicity was shown between three references included (χ2 = 7.60, P = 0.02, I2 = 74%), a random-effect model was used. As compared to the stage IIIb/c group, the 5-year disease-free survival rate was significantly lower (RR = 0.65; 95%CI: 0.40–1.05; Z = 1.75, P = 0.08), as illustrated in Fig. 4.
Fig. 4.
Meta analysis results of 5-year disease-free survival rate in SLNM group vs. stage IIIb/c breast cancer group.
Publication bias
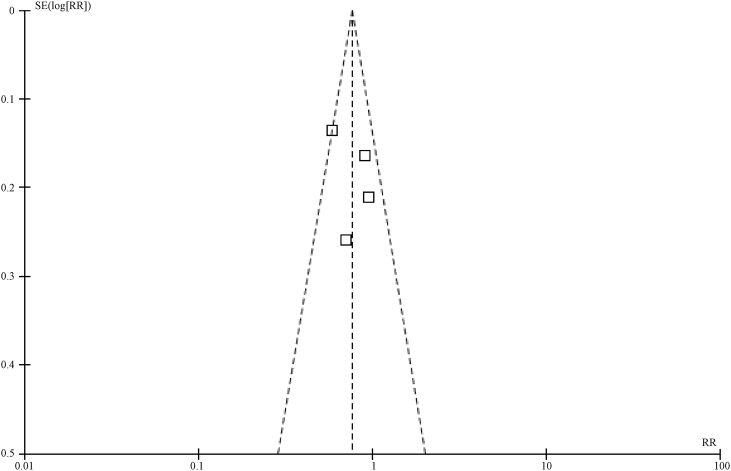
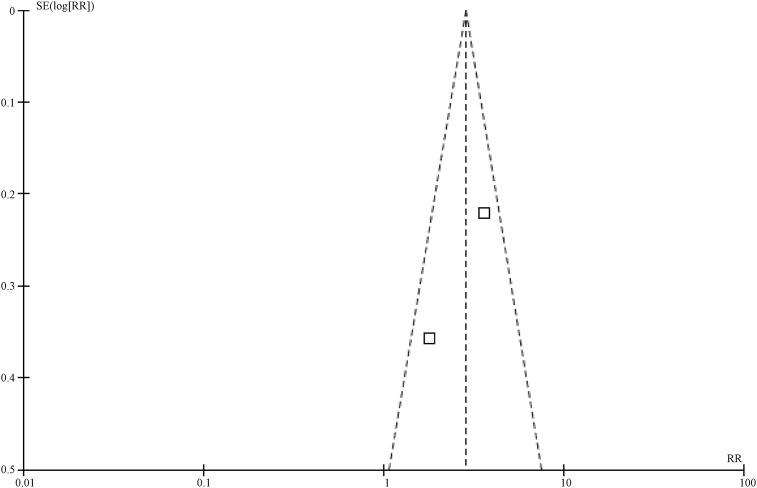
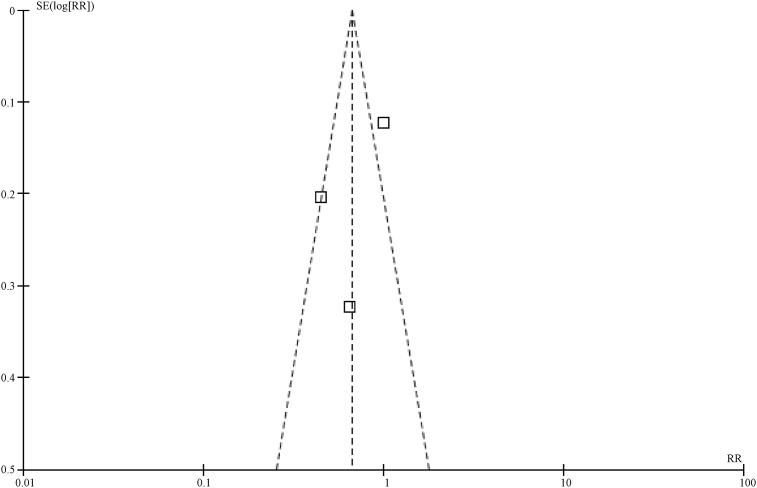
In this study, a funnel plot was used to analyze the two outcome variables (5-year overall survival rate and 5-year disease-free survival rate). As a result, asymmetry was shown in the funnel plot for the influences on 5-year overall survival rate, indicating a potential publication bias (Fig.5, Fig.6). However, the funnel plot for the influences on 5-year disease-free survival rate indicates a low potential for a publication bias (Fig.7).
Fig. 5.
Funnel plot for 5-year survival rate in SLNM group vs. stage IIIb/c breast cancer group.
Fig. 6.
Funnel plot for 5-year survival rate in SLNM group vs. stage IV breast cancer group.
Fig. 7.
Funnel plot for 5-year disease-free survival rate in SLNM group vs. stage IIIb/c breast cancer group.
Discussion
Results of this Meta analysis suggest that, after combined therapy, both the 5-year survival rate and 5-year disease-free survival rate were lower in breast cancer patients with ipsilateral SLNM without distant metastasis than in those with stage IIIb/c breast cancer; although the outcome showed no statistical significance, the trend of the data was on the decline. In contrast, the 5-year survival rate was significantly higher than in those with stage IV breast cancer and the difference was statistically significant. A random-effect model was used for analysis of the prognostic indicators in this meta-analysis because of the heterogenicity between the studies included. The methodology assessment of reference quality suggests that the references tend to have intermediate or low quality, which might be primarily attributable to the difficulty in blinding the treatment regimens. Other limitations of this study mainly lie in that they are lacking in analysis of the results of the long-term prognosis and outcome indicators; some of the studies have an overly small sample size; and that the funnel plot indicates a certain publication bias in this study.
Brito et al5 initially proposed that the patients with ipsilateral SLNM would present with a prognosis that is more similar to that of patients with locally advanced breast cancer than of the patients with distant metastasis. Subsequently Chen,11 Huang,15 Fan,16 Park,17 Ogino,12 Dellapasqua13 and Noh14 all successively reached similar conclusions. The rationality of classifying breast cancer with ipsilateral SLNM as stage N3c instead of M1 in the 6th edition of AJCC-TNM breast cancer staging system was evaluated by the treatment status in these patients. Meanwhile, results of this meta-analysis suggest a significantly different prognosis in breast cancer patients with ipsilateral SLNM from stage M1 patients and a similar prognosis to stage N3c patients. However, though the results of comparing to the stage N3c group were no statistically significant (a possible reason is the small sample size), we could see the differences in the data with a slightly lower 5-year OS and DFS. Therefore, the treatment regimen for breast cancer patients with ipsilateral SLNM should be different from those for stage N3c and stage M1. Chen et al11 argue that good cervical lymph node dissection or other cervical controls, including chemotherapy, could result in significantly improved patient prognosis and survival; this however remains to be confirmed by large-sample, multi-center studies.
Conclusion
In conclusion, this study suggests that the prognosis for breast cancer patients with ipsilateral SLNM is similar to patients with stages IIIb/c disease and different from patients with stage IV disease. The 5-year OS and DFS of the breast cancer patients with ipsilateral SLNM is slightly lower compared with that of the stage IIIb/c group, and radical, instead of palliative therapy, seems more plausible for these patients. In addition, vigilance should be exercised for the high risk of distant metastasis in these patients, requiring a combined therapeutic approach including radical surgery, radiotherapy and chemotherapy, and endocrine therapy, etc. In order to further promote the clinical application of this effective approach, large-sample, multi-center studies with high quality are necessary to improve the evidential robustness of these observations.
Acknowledgments
This study was supported by the grants from the National Natural Science Foundation of China (No. 81372811) and the Science and Technology Agency of Liaoning Province (No. 2013225049).
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Chen S.C., Chen M.F., Hwang T.L. Prediction of supraclavicular lymph node metastasis in breast carcinoma. Int J Radiat Oncol Biol Phys. 2002;52:614–619. doi: 10.1016/s0360-3016(01)02680-3. [DOI] [PubMed] [Google Scholar]
- 2.Debois J.M. The significance of a supraclavicular node metastasis in patients with breast cancer. A literature review. Strahlenther Onkol. 1997;173:1–12. doi: 10.1007/BF03039187. [DOI] [PubMed] [Google Scholar]
- 3.Jackson S.M. Carcinoma of the breast the significance of supraclavicular lymph node metastases. Clin Radiol. 1966;17:107–114. doi: 10.1016/s0009-9260(66)80066-1. [DOI] [PubMed] [Google Scholar]
- 4.Kiricuta I.C., Willner J., Kolbl O., Bohndorf W. The prognostic significance of the supraclavicular lymph node metastases in breast cancer patients. Int J Radiat Oncol Biol Phys. 1994;28:387–393. doi: 10.1016/0360-3016(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 5.Brito R.A., Valero V., Buzdar A.U. Long-term results of combined-modality therapy for locally advanced breast cancer with ipsilateral supraclavicular metastases: the University of Texas M.D. Anderson Cancer Center experience. J Clin Oncol. 2001;19:628–633. doi: 10.1200/JCO.2001.19.3.628. [DOI] [PubMed] [Google Scholar]
- 6.Greene F.L.P.D., Fleming I.D., Fritz A.G. 6th ed. Springer-Verlag; New York: 2002. AJCC Cancer Staging Manual. [Google Scholar]
- 7.Singletary S.E., Allred C., Ashley P. Revision of the American joint committee on cancer staging system for breast cancer. J Clin Oncol. 2002;20:3628–3636. doi: 10.1200/JCO.2002.02.026. [DOI] [PubMed] [Google Scholar]
- 8.Moher D., Liberati A., Tetzlaff J., Altman D.G., Grp P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Wells G., Shea B., O'connell D. 2000. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses. [Google Scholar]
- 10.Higgins JPT GSe. Cochrane Handbook for Systematic Reviews of Interventions Version 5. 1. 0 [DB/OL]. Available from: http://www.cochrane.org/handbook.
- 11.Chen S.C., Chang H.K., Lin Y.C. Prognosis of breast cancer after supraclavicular lymph node metastasis: not a distant metastasis. Ann Surg Oncol. 2006;13(11):1457–1465. doi: 10.1245/s10434-006-9012-1. [DOI] [PubMed] [Google Scholar]
- 12.Ogino T., Komoike Y., Ishitobi M. Breast cancer with ipsilateral supraclavicular metastases. Breast J. 2011;17:555. doi: 10.1111/j.1524-4741.2011.01141.x. e57. [DOI] [PubMed] [Google Scholar]
- 13.Dellapasqua S., Bagnardi V., Balduzzi A. Outcomes of patients with breast cancer who present with ipsilateral supraclavicular or internal mammary lymph node metastases. Clin Breast Cancer. 2014;14(1):53–60. doi: 10.1016/j.clbc.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Noh J.M., Kim K.H., Park W. Prognostic significance of nodal involvement region in clinical stage IIIc breast cancer patients who received primary systemic treatment, surgery, and radiotherapy. Breast. 2015 Oct;24(5):637–641. doi: 10.1016/j.breast.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 15.Huang E.H., Strom E.A., Valero V. Locoregional treatment out-comes for breast cancer patients with ipsilateral supraclavicular metastases at diagnosis. Int J Radiat Oncol Biol Phys. 2007;67:490–496. doi: 10.1016/j.ijrobp.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 16.Fan Y., Xu B., Liao Y. A retrospective study of metachronous and synchronous ipsilateral supraclavicular lymph node metastases in breast cancer patients. Breast. 2010;19:365. doi: 10.1016/j.breast.2010.03.022. e69. [DOI] [PubMed] [Google Scholar]
- 17.Park H.J., Shin K.H., Cho K.H. Outcomes of positron emission tomography-staged clinical N3 breast cancer treated with neoadjuvant chemotherapy, surgery, and radiotherapy. Int J Radiat Oncol Biol Phys. 2011;81:e689–e695. doi: 10.1016/j.ijrobp.2010.11.061. [DOI] [PubMed] [Google Scholar]