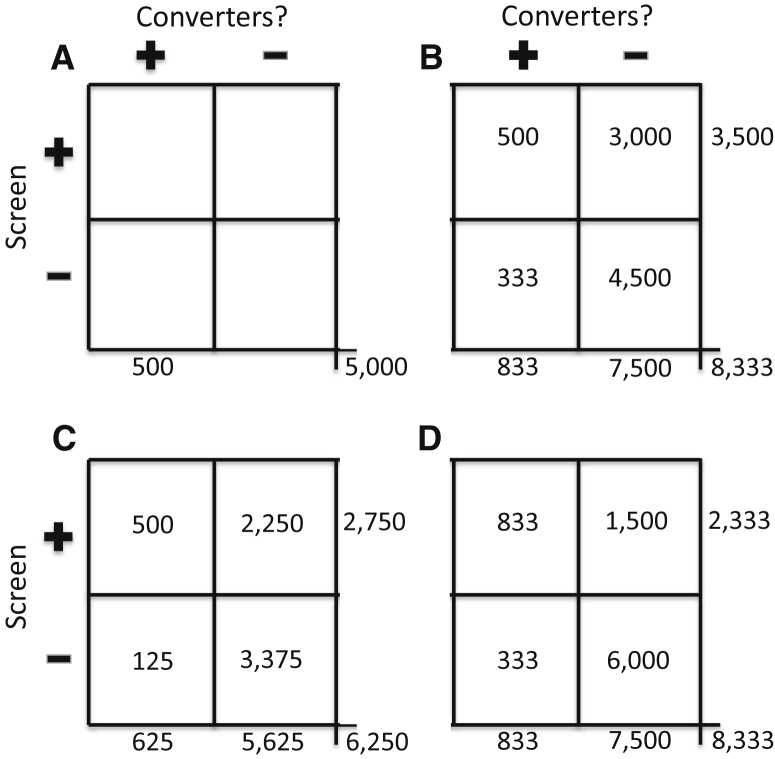
Fig. 1.
Four hypothetical applications of screening methods when selecting participants for an AD/MCI prevention trial in a population with base rate of 10% who will develop symptoms over 5 years of follow-up. (A) shows results with no screening test. (B) represents results expected with a screening test that provides 60% sensitivity and 60% specificity in identification of those who will “convert” during the 5-year trial interval. (C) indicates a degree of improvement expected if sensitivity of the prior method can be improved to 80% with no change in specificity. (D) shows the results expected when specificity is improved to 80% but sensitivity is 60%, as in (B). Implications for the costs of the trial are substantial (see text).