Figure 2. Methods by which splicing may be modulated for cancer therapy.
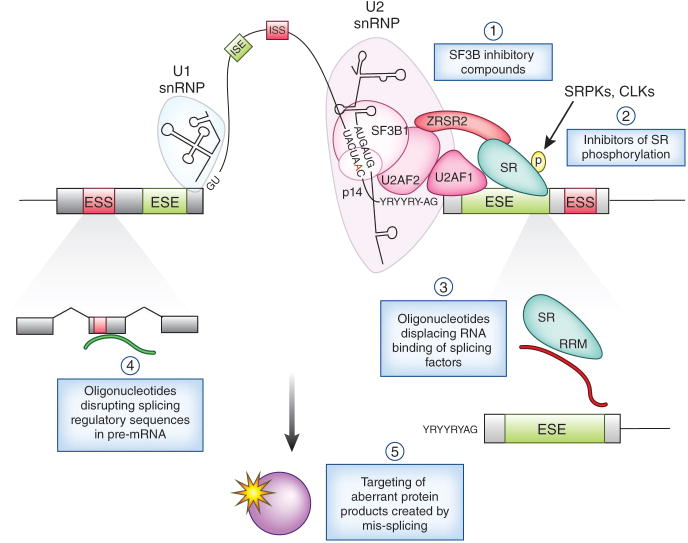
These include (1) use of a series of compounds that inhibit early spliceosome assembly by inhibiting SF3B1 and (2) inhibition of phosphorylation of Serine/Arginine-rich (SR) proteins through inhibition of CLKs (CDC2-like kinases) and/or SRPKs (SR protein kinases). In addition, use of oligonucleotides to (3) target the RNA binding activity of splicing regulatory proteins mutated or overexpressed in cancer, or (4) directly alter splicing of individual downstream mRNA's critical for cancer pathogenesis, may provide more selective tumor targeting. Alteration of specific events may be achieved by oligonucleotides that alter splicing by promoting or impairing the use of key splicing regulatory sequences (such as 5′/3′ splice sites, exonic splicing silencers (ESSs), exonic splicing enhancers (ESEs), intronic splicing enhancers (ISEs), and intronic splicing silencers (ISSs)). In addition, identification of novel proteins stably produced by aberrant splicing in cancer may result in therapeutic strategies to target these downstream pathologic products and pathways (as depicted in (5)). Additional therapeutic strategies that have been shown to affect splicing only in cell-free in vitro assays to date are not shown above. Inhibition of U2 snRNP by inhibition of SF3B1 function or methylation of Sm proteins is shown in more detail in Figure 3.
