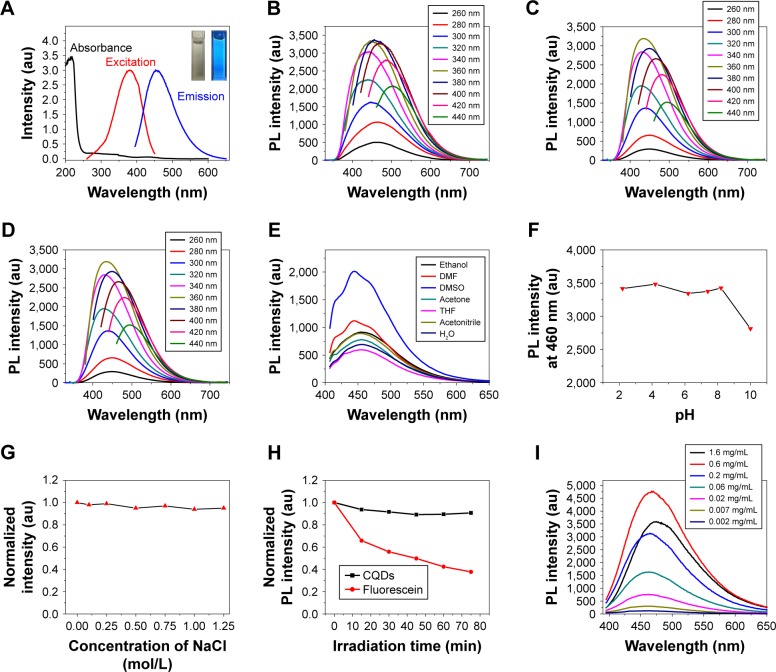
Figure 6.
(A) UV-vis absorption, excitation, and fluorescence spectra of CQDs (the right inset is the photograph of CQDs under 365 nm excitation in comparison to the blank control [left inset]); Fluorescence spectra of (B) CQDs, (C) Pd-CQDs, and (D) Pt-CQDs with different excitation wavelengths; (E) fluorescence spectra of CQDs in various solvents; (F) effect of pH value on the PL intensity of the CQDs aqueous solution at 460 nm; (G) effect of concentration of NaCl on the PL intensity of the CQDs aqueous solution at 460 nm; (H) comparison on the photobleaching characteristics of CQDs and fluorescein (the samples were continuously irradiated using a 500 W xenon lamp and the fluorescence intensity was normalized); (I) concentration-dependent behavior of CQDs in aqueous solution.
Abbreviations: UV, ultraviolet; vis, visible; CQDs, carbon quantum dots; PL, photoluminescence; DMF, dimethylformamide; THF, tetrahydrofuran.