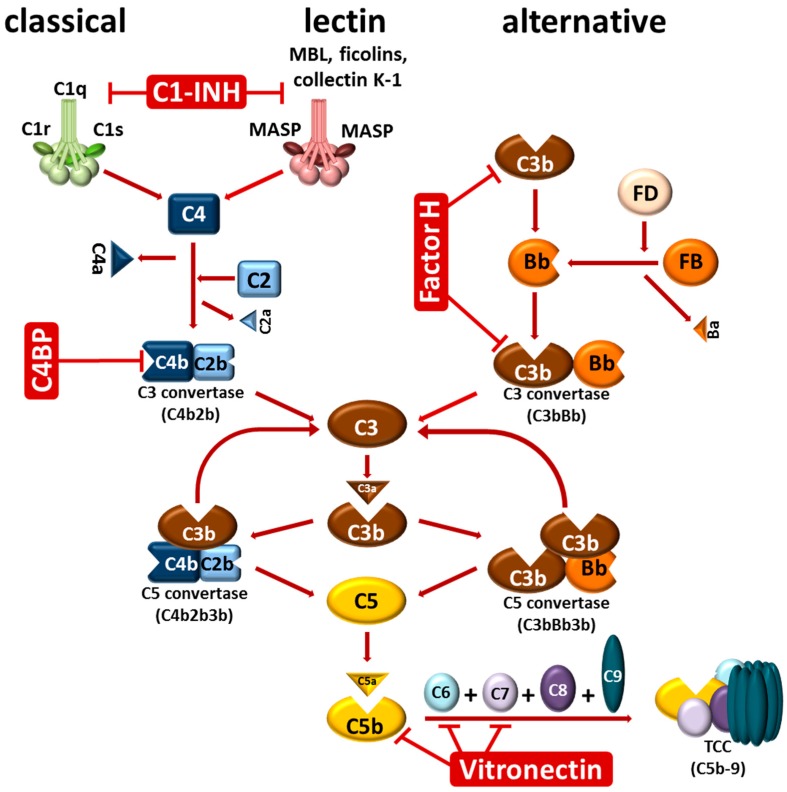
Figure 1.
Activation of the complement system. Complement is activated in a sequential manner by three pathways: the classical, lectin, and alternative pathway. The classical pathway is initiated by binding of the C1 complex consisting of one molecule of C1q, two molecules of C1r, and two molecules of C1s to immunoglobulins (IgM, IgG) bound to their corresponding antigens. Activated C1s cleaves C4 which, upon covalent binding to the target surface, cleaves C2 leading to the formation of the C3 convertase C4b2b of the classical pathway. Activation of the lectin pathway is triggered by binding of mannan-binding lectin (MBL), ficolins (H-ficolin, L-ficolin, M-ficolin) or collectin K-1 associated with MBL-associated serine proteases MASPs to a variety of carbohydrates of microbial origin. MASP-1 and MASP-2 (MASP) are able to cleave C4 and then C2 to form the same C3 convertase. The alternative pathway is initiated by covalent binding of C3b molecules to foreign particles (opsonization). Surface-bound C3b molecules recruit Factor B (FB) leading to the formation of a C3bB complex. Following cleavage of FB by Factor D (FD), the C3 convertase (C3bBb) of the alternative pathway is generated. This enzyme cleaves C3 molecules into the small C3a fragment (anaphylatoxin) and C3b which covalently binds in close vicinity of the newly formed C3 convertases, thereby resulting in a strong amplification loop that generates an increasing number of highly reactive C3b molecules. Binding of C3b to C4b and C3b within the C3 convertase of the classical and alternative pathway leads to the formation of the C5 convertase (C4b2b3b and C3bBb3b). Binding of the additional C3b molecule changes the substrate specificity of these enzymes towards C5 which subsequently is cleaved into C5b and the most potent anaphylatoxin, C5a. Generation of C5b initiates the activation of the terminal pathway by sequential binding of C6, C7, C8, and C9 to C5b. Upon binding of multiple C9 molecules (C9 polymerization) the pore-forming terminal complement complex (TCC) integrates into the membrane and leads to lysis of susceptible cells. The soluble regulators that control activation at the level of complement initiation (C1-INH) act as cofactors for FI-mediated inactivation of C3b (Factor H) or C4b (C4BP), and prevent formation of the TCC and integration of the complex into the membrane (vitronectin).