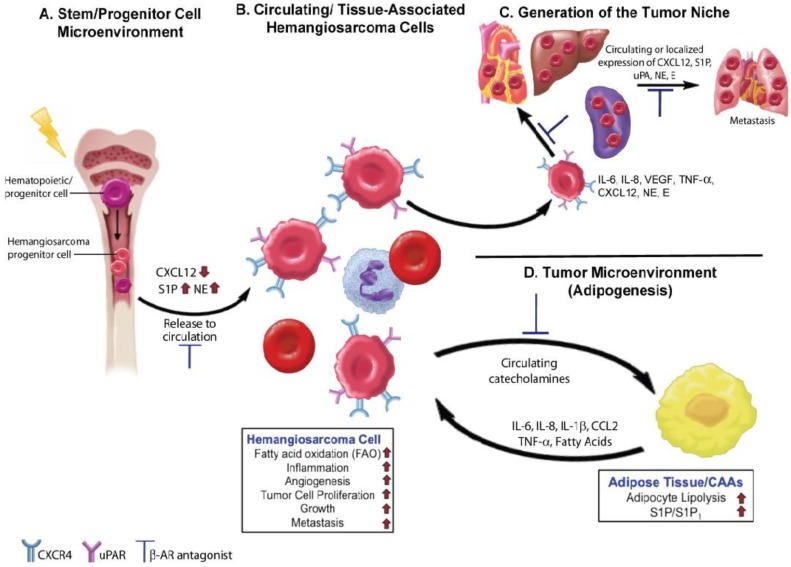
Figure 2.
Beta adrenergic signaling drives essential processes in tumor initiation and progression in canine hemangiosarcomas and human angiosarcomas. (A) Norepinephrine (NE) is released by the sympathetic nervous system in the bone marrow, where it binds to β-AR and causes a decrease in the release of CXCL12 within hematopoietic stem cell niches. Reductions in CXCL12 levels lead to increased hematopoiesis. In hemangiosarcomas, DNA damaging events may occur at the level of hematopoietic stem or progenitor cells leading to the generation of hemangiosarcoma progenitor cells. Increased levels of NE, might then promote the release of hemangiosarcoma cells into the circulation. Inhibition of NE activity by β-AR antagonists could limit disease progression by restoring CXCL12 levels and regulation of the bone marrow microenvironment; (B) Hemangiosarcoma cells released into the circulation may exist as progenitor cells or more differentiated tumor cells. The hemangiosarcoma cells express CXCR4 receptors, which promote tumor cell homing to tissue sites that express high levels of CXCL12. S1P may also be produced by RBCs and platelets in the circulation and in tissues to further promote homing and tumor cell dissemination; (C) The secretion of proangiogenic and proinflammatory factors by hemangiosarcoma cells promotes the formation of the tumor cell niche. NE and epinephrine (E) released locally by sympathetic nerve fibers or found circulating in the blood may further activate tumor cells and promote tumor initiation and growth; (D) Tumor cells possess the ability to hijack the metabolic processes of normal cells, such as adipocytes, present within the surrounding local environment. Activation of β-AR on adipocytes by locally produced or circulating NE and E can lead to the further production and release of angiogenic and inflammatory factors, promoting tumor growth. Upon beta adrenergic stimulation, adipocytes release fatty acids into the tumor microenvironment, which can then serve as a metabolic fuel for tumor cells or for the production of essential metabolites needed for efficient tumorigenesis. Potential areas in this process where β-AR antagonists may slow or halt tumorigenesis are indicated.