Abstract
Activating mutations of the BRAF gene lead to constitutive activation of the MAPK pathway. The characterization and discovery of BRAF mutations in a variety of human cancers has led to the development of specific inhibitors targeting the BRAF/MAPK pathway and dramatically changed clinical outcomes in BRAF-mutant melanoma patients. Recent discovery of BRAF mutation in canine cancers underscores the importance of MAPK pathway activation as an oncogenic molecular alteration evolutionarily conserved between species. A comparative approach using the domestic dog as a spontaneous cancer model will provide new insights into the dysregulation of BRAF/MAPK pathway in carcinogenesis and facilitate in vivo studies to evaluate therapeutic strategies targeting this pathway’s molecules for cancer therapy. The BRAF mutation in canine cancers may also represent a molecular marker and therapeutic target in veterinary oncology. This review article summarizes the current knowledge on BRAF mutations in human and canine cancers and discusses the potential applications of this abnormality in veterinary oncology.
Keywords: dogs, comparative oncology, bladder cancer, urothelial carcinoma, transitional cell carcinoma, mitogen-activated protein kinase
1. BRAF/MAPK Pathway in Cancer Pathogenesis
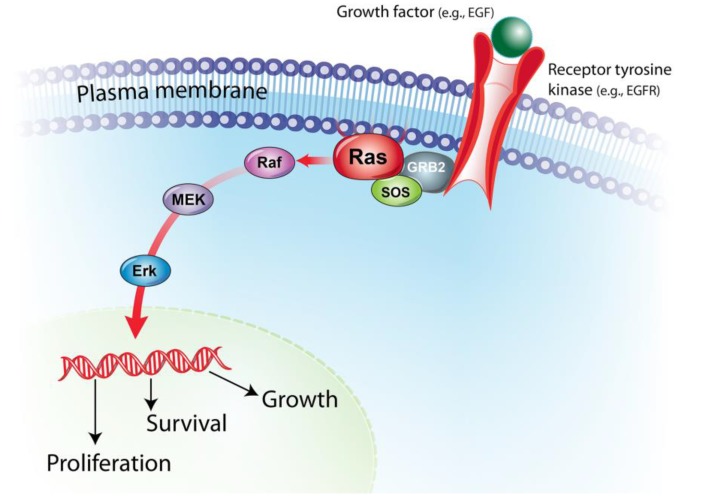
The mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathway is an evolutionary conserved molecular pathway that regulates fundamental cellular processes, including cell growth, proliferation, differentiation and apoptosis. MAPK pathway signaling is initiated by many different extracellular signals such as growth factors and mitogens. Ligand binding to receptor tyrosine kinases (e.g., EGF and its receptors) triggers phosphorylation and activation of RAS families, which in turn activates RAF proteins. Activation of RAF leads to subsequent activation of MEK, initiating the signal transduction of many genes involved in different cellular processes [1] (Figure 1).
Figure 1.
Mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathway. The MAPK/ERK signaling pathway is activated by many different extracellular signals such as binding of growth factors (e.g., EGF) to its receptors (e.g., EGFR). Activated receptor tyrosine kinase phosphorylates and activates RAS family proteins through GRB2-SOS adaptor protein complex. Activated RAS protein, in turn, activates the serine/threonine kinase function of RAF proteins. RAF phosphorylates MEK, which phosphorylates and activates ERK, initiating the signal transduction of many genes involved in various cellular processes.
The MAPK pathway is activated in many cancers through different molecular mechanisms, enabling cancer cells to grow independently of extracellular proliferation signals. Somatic mutation of RAS genes is one molecular alteration leading to constitutive activation of the MAPK pathway. Activating mutations of three RAS genes, HRAS, KRAS and NRAS, are found in 20%–25% of all human cancers [2]. Similarly, RAS mutations are present in canine cancers including lung cancer [3], leukemia [4] and other types of cancer [5,6,7].
Activating mutations of RAF genes represent another mechanism for constitutive activation of the MAPK pathway. The RAF family consists of three members: ARAF, BRAF and CRAF. Among the three forms of RAF genes, BRAF gene is most frequently mutated in human cancer [1,8,9]. Since the discovery of BRAF mutations in melanoma and other cancers in 2002 [10], a number of studies further identified and characterized BRAF mutations in human cancer. The most common (>90%) somatic mutation of the human BRAF gene is a T-to-A transversion in exon 15 at nucleotide 1799 (c.1799T > A), resulting in the amino acid substitution from valine to glutamic acid at codon 600 (V600E) [1]. The BRAF V600E (BRAFV600E) mutation occurs within the activation segment of the gene and mimics phosphorylation, drastically elevating kinase activity and activating downstream signal [9,10]. The discovery of BRAF mutations and MAPK pathway dependence of human cancers led to the therapeutic strategy targeting BRAF/MAPK pathway.
BRAF exon 15 is highly conserved between species; amino acid sequences encoded by exon 15 are identical between humans and dogs, including valine at codon 600 in human BRAF. Two recent studies identified canine BRAF V595E mutation, a somatic mutation of canine BRAF gene orthologous to human V600E, in different types of canine cancers [11,12] (note: this mutation is a T to A transversion at position 8,296,284 on dog chromosome 16 (canFam3.1). Previous studies referred to this mutation as either V595E or V450E, due to the use of different reference sequences. Throughout this paper we use “V595E” to avoid confusion, with a protein sequence based on Ensemble Transcript ID:ENSCAFT 00000006306). Coupled with frequent mutations of RAS genes between human and canine cancers, the evolutionarily conserved BRAF mutations underscore the importance of MAPK pathway activation as a common oncogenic molecular pathway. This review article summarizes the current knowledge of BRAF mutations in human and canine cancers and discusses potential applications of the dysregulation of BRAF/MAPK pathway in veterinary oncology.
2. BRAF Mutations in Human and Canine Cancers
2.1. Melanocytic Tumors
Perhaps the most well described BRAF-mutated cancer in humans is melanoma. Melanoma is a cancer originating in melanocytes, occurring mainly in skin (>90%), but also in other locations including eye and mucosal regions [13,14]. Cutaneous melanoma is the fifth and seventh most commonly diagnosed cancer in men and women, respectively, with diagnosis in >70,000 cases and ~10,000 death in the US each year [15]. Furthermore, incidence of cutaneous melanoma has been continuously growing in the Western countries over the past three decades as much as fivefold [16]. Constitutive activation of MAPK signaling plays an important role in the pathogenesis of human melanoma through activating mutations of BRAF (~60%) or NRAS (~15%) genes [10,17,18,19]. The V600E mutation is the most common form of the BRAF mutation in human melanoma [17].
Malignant melanoma is the most common neoplasm of the oral cavity in dogs, but also occurs on the skin, digits and eye [20,21]. As in human melanoma, constitutive activation of the MAPK pathway is also implicated in canine melanoma [22,23]. Several studies have examined the presence of BRAF mutations in canine melanoma; however, only one study identified cBRAFV595E mutations in a small percentage of patients (6%) [11,21,22]. The disparity in the prevalence of BRAF mutation may result from differences in the role of UV exposure in the pathogenesis of human and canine melanoma. In human melanoma, the presence of BRAF mutations is associated with UV exposure, and tumors on mucosal sites or non-UV-exposed skin rarely possess the mutation [24,25]. Unlike humans, the furred-skin of dogs provides natural protection from UV damage. This protection from UV may make dogs less susceptible to UV-related melanoma, resulting in differences in the anatomical location of melanoma between species; the cutaneous form accounts only for ~25% of canine melanoma, with the majority of tumors arising in the oral cavity [21]. The low frequency of BRAF mutations among canine melanomas, coupled with UV-independent carcinogenesis and unique anatomical distribution, supports the role of the dog as a spontaneous model for investigation of the BRAF-independent pathogenesis of non-UV-associated melanoma, a rare subtype of human melanoma.
It is noteworthy that benign melanocytic lesions also harbor BRAF mutations, in both humans and dogs, with frequencies similar to those of malignant melanoma. The BRAF mutation was found in 82% of nevi in humans and in 17% of canine melanocytomas [11,26], suggesting the BRAF mutation and consequent MAPK activation may play an important role in the initiation of melanocytic neoplasms, but may be insufficient to cause malignant melanoma without additional molecular alterations.
2.2. Urothelial Carcinoma and Prostatic Carcinoma
Urothelial carcinoma (UC), also known as transitional cell carcinoma, is the most common (>90%) form of human bladder cancer. Human UC is subdivided into two distinct entities based on the extent of tumor invasion: non-muscle-invasive bladder cancers (NMIBCs) and muscle invasive bladder cancers (MIBCs). NMIBCs carry a more favorable prognosis with five-year survival of ~90%, while local and distant metastasis is common in MIBCs. MIBCs show more complex genomic alterations including chromosomal aneuploidity, chromothripsis and frequent mutations of the TP53 gene, reflecting their aggressive biological behavior [27]. Although the BRAF mutation is uncommon in human UC [28,29], the MAPK pathway activation through different molecular alterations is implicated in human UC, especially in NMIBCs. Somatic mutations in genes upstream of the MAPK pathway, including HRAS, KRAS and FGFR3 genes, were found in ~80% of NMIBCs and ~40% of MIBCs in a mutually exclusive manner, suggesting that mutations of these genes lead to activation of the same pathway [30]. Normal human urothelial cells gain proliferation and survival advantage through FGFR3 mutations and subsequent MAPK pathway activation [31]. Coupled with the fact that mutations of FGFR3 gene are more common in NMIBCs, activation of the MAPK pathway may be a fundamental molecular alteration in the initiation of NMIBCs.
Canine UC is the most common malignancy in the lower urinary tract, accounting for ~1%–2% of cancer in this species. Definitive diagnosis of canine UC is made by histological examination of tissue specimens obtained by cystoscopy or surgical biopsy [32]. Although it is often difficult to assess the extent of tumor invasion due to the superficial nature of specimens obtained by cystoscopy, the majority of canine UCs are considered invasive with >90% of tumors invading the bladder wall and 20% having metastasis at the time of diagnosis [33]. Two recent studies identified the cBRAFV595E mutation in 87% and 67% of canine UC cohorts [11,12]. Similar to the human BRAFV600E mutation, the cBRAFV595E results in activation of the MAPK pathway, which can be reversed by a BRAF inhibitor [12]. Given the high incidence of the BRAF mutation in canine UC, therapy targeting the BRAF/MAPK pathway may thus offer a novel treatment option for dogs with BRAF-mutated UC.
As in the case of UC, cBRAFV595E was found in 80% of prostatic carcinoma (PC) in dogs [11], but is an uncommon mutation in human PC [34,35]. It is interesting that canine UC and PC share the BRAF mutation at similar frequencies. This shared molecular alteration may, however, imply that the majority of canine PCs arise from the urothelium. The cellular origin of “carcinoma of the prostate gland” is controversial in dogs. Several studies demonstrated that canine PC shows highly variable morphological characteristics, some of which resemble UC, complicating histopathological distinction between PC and UC arising from prostatic urethra [36,37]. Immunohistochemical markers also fail to differentiate these two cancers, as canine PC cells express urothelial markers [38,39]. Considering the androgen-independent nature of canine PC, it is now believed that the majority of canine PC originates from prostatic ducts and/or prostatic urethra. The similar frequencies of cBRAFV595E in canine PC and UC also support this hypothesis. Taken together, the high incidence rates of cBRAFV595E underscore the importance of BRAF/MAPK pathway in the pathogenesis of UC and PC and may present dog as a suitable model for BRAF/MAPK pathway-targeted therapy for human UC and PC.
2.3. Brain Tumors
Meningioma and glioma are two major histological types of primary intracranial malignancies in both humans and dogs. Meningioma is a tumor that arises from meninges, whereas glioma is a broad category of brain tumors originating from glial cells including glioblastoma, astrocytoma, oligodendroglioma and ependymoma. These tumors are further subdivided into each histological subtype [40]. Genetic alteration of the BRAF gene and subsequent MAPK pathway activation is a frequent molecular event in a subset of human glioma, astrocytoma. The BRAF gene is altered by a missense mutation (mainly V600E) or tandem duplication of BRAF locus resulting in the KIAA1549:BRAF fusion gene in up to 70% of astrocytoma (especially in picocytic astrocytoma and pleomorphic xanthoastrocytoma), while such alteration is uncommon in other types of glial and non-glial tumors [41,42,43,44]. The identification of BRAF/MAPK dysregulation has promoted the potential use of BRAF inhibitor therapy in neuro-oncology [45,46].
Similar to human brain tumors, cBRAFV595E has been detected in 15% of canine gliomas, and was undetected in 20 cases of meningioma [11]. Although only 15% of canine gliomas harbored a detectable cBRAFV595E, it is of note that ~60% of canine gliomas show copy number gain of the gene, which raises the possibility that increase in BRAF gene dosage may serve as another mechanism for MAPK pathway dysregulation [47]. The presence of BRAF alterations in canine glioma, coupled with anatomical and physiological similarities between canine and human brain tumors, offer further indication that studies of these tumors in dogs may serve as a relevant model to explore the therapy targeting BRAF/MAPK pathway in neuro-oncology.
2.4. Hematopoietic Tumors
Mutation of the BRAF gene is rare in human common hematopoietic cancers including Non-Hodgkin’s lymphoma [48,49], multiple myeloma [50] and acute and chronic leukemia of lymphoid and myeloid origins [48,51]. Recently, the BRAFV600E mutation was found in significant proportions of two rare hematopoietic malignancies: Langerhans cell histiocytosis (LCH) and Hairy-cell leukemia (HCL). LCH is a clonal proliferative disease of Langerhans cells, the epidermal antigen-presenting cells. A rare hematologic malignancy, HCL is characterized by expansion of abnormal B cells in bone marrow and spleen. The BRAF mutation was detected in 57% of LCH [52] and 100% of HCL [48], all of which were BRAFV600E. The high prevalence of BRAFV600E in two human hematopoietic malignancies led researchers to investigate the presence of BRAF mutations in canine hematopoietic cancers; however, BRAF mutations were not detected in any of 245 canine hematopoietic cancers including tumors of histiocytic (histiocytic sarcoma and histiocytoma), lymphoid (lymphoma, plasmacytoma and acute and chronic lymphocytic leukemia), myeloid (acute myelogenous leukemia) and mast cell (mast cell tumor) origins [11]. This absence of BRAF mutations in canine hematopoietic cancers may reflect the fact that dogs do not develop diseases that are the counterpart of human LCH or HCL, or perhaps that the MAPK pathway is activated by different molecular mechanisms such as alterations of RAS or receptor tyrosine kinases [4,53,54,55,56].
2.5. Thyroid Cancers
The activation of the MAPK pathway, as well as PI3K/AKT pathways, is crucial for the initiation and progression of human thyroid cancers [57]. The BRAF mutation has been detected in 45% of papillary thyroid carcinoma (PTC) and in 24% of atypical subtype, whereas the mutation has not been detected in follicular and medullary thyroid carcinoma (FTC and MTC, respectively) [58].
Thyroid cancer is the most common endocrine tumor in dogs, with 90% of tumors being malignant [59]. The difference in histopathological distribution of human and canine thyroid cancers exists; the most common histological subtype of thyroid cancer in dogs is FTC, whereas PTC is the most common form in humans [60]. One study examined the presence of BRAF mutations in a cohort of canine thyroid cancers comprising 47 FTC and 16 MTC. Although there was no evidence of BRAF mutations in the cohort, the same study demonstrated the upregulation of PI3K/AKT pathway molecules in canine thyroid cancers, consistent with human FTC and MTC [5,57]. Absence of BRAF mutation and upregulated PI3K/AKT pathway in canine thyroid cancers suggest that activation of PI3K/AKT pathway, rather than MAPK pathway, plays a more important role for the tumorigenesis of FTC and MTC.
2.6. Other Cancers
BRAF mutation is a common genetic alteration in human epithelial cells including lung and colorectal carcinomas, with frequencies of up to 20%, whereas sarcomas rarely possess the mutation [10,17]. This holds true for canine cancers, where the BRAF mutation was detected in pulmonary carcinoma and oral squamous cell carcinoma, but absent in common types of canine sarcoma, including hemangiosarcoma, osteosarcoma and soft tissue sarcoma [11]. The prevalence of the BRAF mutation has not been characterized in less common canine epithelial tumors, such as carcinomas of the liver and gastrointestinal tract. In addition, since BRAF mutations outside of exon 15 has been poorly investigated in canine malignancies, specific types of canine cancer may harbor BRAF mutations in other exons as in the case of human lung carcinoma, where 10%–30% of BRAF mutations are located in exon 11 [61,62]. The detection of BRAF mutation in canine malignancies is still ongoing, and a characterization of BRAF mutations across canine cancers will not only provide further insights into oncogenic roles of BRAF alteration in different types of cancers but also lead to a new diagnostic and therapeutic strategy for BRAF-mutant canine cancers.
3. Clinical Implications of BRAF Mutations in Human and Canine Oncology
3.1. BRAF Mutation as a Cancer Marker
Detection of BRAF mutations has formed the basis of molecular methods of diagnostics and disease monitoring, using DNA isolated from biopsy material, either from surgical biopsy or fine needle aspirates. One example for such attempt to use BRAF mutations as a molecular testing is clinical management of thyroid cancer. Thyroid nodules are common lesions found in 4%–7% of the adult population [63]. As most nodules are benign, only 5% are cancerous and require surgical intervention. Cytological examination of nodules is the first step to rule out malignancies. However, inconclusive cytological results often lead to unnecessary diagnostic lobectomy of benign nodules. Patients whose nodules were found to be malignant by diagnostic lobectomy also have to undergo a second surgery for thyroidectomy. To avoid unnecessary surgeries for benign nodules and the two-step surgical management for thyroid cancers, the use of molecular markers has been proposed as an aid for preoperative diagnosis [57]. As nodules with the BRAF mutation are ~100% indicative of thyroid cancers (PTCs), BRAF-mutated thyroid nodules can be treated by total thyroidectomy without necessitating the diagnostic lobectomy [57,58]. Although the BRAF mutation alone is not sensitive enough to detect majority of thyroid cancers, a test to detect other molecular abnormalities in combination with BRAF mutations were found to increase the sensitivity [64].
Recent advancement of genome technology makes it possible to now detect low numbers of mutant sequences in blood-derived cell-free DNA as a means of disease monitoring and molecular profiling without biopsy of primary tumor (called “liquid biopsy, reviewed in [65]). The detection and/or quantification of circulating BRAF mutant alleles is correlated with presence of metastasis, drug response and clinical outcomes in melanoma patients, suggesting that circulating detection of a BRAF mutation can be a non-invasive molecular marker for disease monitoring and treatment selection [66,67].
In veterinary medicine, a major challenge in the clinical management of canine UC and PC lies in early and accurate diagnosis, as these cancers are often diagnosed at advanced stage [32,68]. Currently, reliable diagnostic tests for PC and UC are limited to histopathological examination of a tissue, which involves general anesthesia and expensive procedures such as cystoscopy and a surgical biopsy of bladder or prostate. Although cystoscopy is considered a less invasive and preferable method for UC tissue collection, cystoscopy may not be applicable to all dogs, depending on their size and sex as well as availability of the equipment [69]. In addition, tissues obtained through cystoscopy are generally small and sometimes pose a diagnostic challenge to pathologists, underscoring the necessity of access to a diagnostic means that can detect even a small number of tumor cells. A potentially highly sensitive molecular assay for the detection of malignant epithelial cells in free catch urine has been developed using fluorescence in situ hybridization (FISH) to detect numerical chromosomal change in UC tumor cells [70]. Coupled with the FISH analysis, the unique high incidence rates of BRAF mutation in canine PC and UC suggest that this mutation may serve as a potential cancer marker for these diagnostically challenging cancers. Since UC and PC cancer cells often shed into urine [32,68,71], detection of the canine BRAF mutation in urine offers utility as another non-invasive molecular diagnostics for canine UC and PC.
Although the BRAF mutation can be a promising molecular marker for these cancers, detection of the mutation in urine may be challenging due to several reasons. First, it is expected that neoplastic cells represent only a minor fraction of nucleated cells in urine. Secondary cystitis is common in dogs with UC and PC, resulting in dilution of the tumor cell population by inflammatory cells in urine [32,72]. One study demonstrated that the cBRAFV595E mutation was detectable in urine samples of dogs with UC by next generation sequencing of a targeted PCR amplicon; however, the mutation was not detected in some urine samples when the same samples were analyzed by PCR-RFLP, a less sensitive method for mutation detection, suggesting that employment of a sensitive method is a key for the mutation detection in urine samples [12]. Another technical challenge is the existence of PCR inhibitors in urine-derived DNA. It is well-known that PCR inhibitors are co-purified when isolating DNA from urine, compromising PCR efficiencies at various degrees [73,74]. This is most problematic when detection assays rely on PCR efficiency (e.g., quantitative PCR), as the presence of PCR inhibitors could lead to underestimation of target concentrations or false-negative results, especially for targets of a small number of copies. Molecular techniques more resistant to PCR inhibitors, such as digital PCR [75], may need to be employed for reliable detection of cBRAFV595E in urine samples.
3.2. BRAF/MAPK-Targeted Therapy
Discovery of BRAF mutations in a wide variety of human cancers opened a new era of BRAF/MAPK targeted therapy for BRAF-mutant cancers. In particular, efforts have been focused on targeting mutant BRAF for treatment in patients with metastatic melanoma. Prognosis for patients with stage IV disease (tumors with distant metastasis) is dismal with one-year survival rate of 33%–62%, due to limited effective treatment options [76]. After the discovery and characterization of BRAF mutations in human melanoma, vemurafenib, the first selective inhibitor for mutant BRAF, was developed and evaluated for a treatment option for patients with BRAF-mutated metastatic melanoma. In the phase III clinical trial, objective response rates in metastatic melanoma with BRAF mutations were 48% for vemurafenib and 5% for dacarbazine, the gold standard treatment for metastatic melanoma at that time, resulting in a significant difference in survival rates at six months (84% in the vemurafenib group vs. 64% in the dacarbazine group) [77]. After demonstrating its efficacy, vemurafenib was approved for the treatment in patients with BRAF-mutated melanoma by the US Food and Drug Administration (FDA) in 2011. To date, in addition to vemurafenib, several drugs targeting the BRAF/MAPK pathway have demonstrated promising clinical efficacies for BRAF-mutated melanoma [78,79]. The BRAF/MAPK-targeted therapy has also shown therapeutic potentials in other BRAF-mutated human malignancies [80,81,82].
The presence of orthologous BRAF mutation in canine cancers raises the possibility that targeting BRAF/MAPK pathway may also provide therapeutic benefits for canine cancers with cBRAFV595E, especially canine UC and PC. The presence of a BRAF mutation, however, does not always correlate with clinical response for BRAF inhibitor. In human non-melanoma malignancies, the therapeutic efficacy of BRAF inhibitors is highly variable between different types of cancer, despite the presence of the BRAFV600E mutation [83]. BRAF-mutant cancer cells can negate antitumor effects of BRAF inhibition by activating different signaling pathways for cell survival and proliferation. For example, unlike melanoma, BRAF inhibitor monotherapy showed limited clinical response for patients with colorectal adenocarcinoma (CRC) harboring BRAFV600E [84]. This de novo resistance is caused by reactivation of EGFR signaling in CRC cells by disrupting a negative feedback loop that suppress EGFR signaling [85]. The re-activated EGFR signaling allows CRC cancer cells to proliferate in the presence of BRAF inhibition. A similar bypass mechanism of BRAF inhibition is also reported in thyroid cancer cells, through reactivation of HER2/HER3 signaling [86]. These human cancer studies showed that BRAF mutational status alone does not predict the therapeutic potential of BRAF inhibition for cancers, suggesting that understanding of molecular networks important for cancer cells is crucial. Although a BRAF inhibitor showed anti-proliferative effects for canine UC cancer cells with cBRAFV595E at relatively high dosage [12], further characterization of in vitro and in vivo effects of BRAF inhibition are warranted for the clinical application of the BRAF/MAPK-targeted therapy for cBRAFV595E-mutated canine cancers.
4. Conclusions
Recent discovery of the BRAF mutation cBRAFV595E (orthologous to the human BRAF V600E mutation) in a variety of canine cancers underscores the importance of MAPK pathway activation in carcinogenesis. Using the dog as a relevant spontaneous cancer model, this evolutionarily-conserved molecular alteration may provide a unique opportunity to better understand the oncogenic role of MAPK pathway activation and test molecular-targeted therapies. From a veterinary oncology perspective, high frequencies of the BRAF mutation in canine UC and PC may represent a promising molecular diagnostic marker and therapeutic target for these clinically challenging cancers. Further studies to characterize the BRAF mutation and MAPK pathway dysregulation in canine cancer will benefit both human and veterinary oncology.
Acknowledgements
We would like to acknowledge Susan Shapiro for helpful discussions. Matthew Breen holds the Oscar J. Fletcher Distinguished Professorship in Comparative Oncology Genetics. This work was funded in part by the NCSU Cancer Genomics Fund.
Author Contributions
Conceived and wrote the paper by Hiroyuki Mochizuki and Matthew Breen.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Dhillon A., Hagan S., Rath O., Kolch W. Map kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 2.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 3.Griffey S.M., Kraegel S.A., Madewell B.R. Rapid detection of K-ras gene mutations in canine lung cancer using single-strand conformational polymorphism analysis. Carcinogenesis. 1998;19:959–963. doi: 10.1093/carcin/19.6.959. [DOI] [PubMed] [Google Scholar]
- 4.Usher S.G., Radford A.D., Villiers E.J., Blackwood L. RAS, FLT3, and C-KIT mutations in immunophenotyped canine leukemias. Exp. Hematol. 2009;37:65–77. doi: 10.1016/j.exphem.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Campos M., Kool M.M., Daminet S., Ducatelle R., Rutteman G., Kooistra H.S., Galac S., Mol J.A. Upregulation of the PI3K/AKT pathway in the tumorigenesis of canine thyroid carcinoma. J. Vet. Intern. Med. 2014;28:1814–1823. doi: 10.1111/jvim.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayr B., Schaffner G., Reifinger M. K-ras mutations in canine pancreatic cancers. Vet. Rec. 2003;153:87–89. doi: 10.1136/vr.153.3.87. [DOI] [PubMed] [Google Scholar]
- 7.Richter A., Murua Escobar H., Gunther K., Soller J.T., Winkler S., Nolte I., Bullerdiek J. RAS gene hot-spot mutations in canine neoplasias. J. Heredity. 2005;96:764–765. doi: 10.1093/jhered/esi121. [DOI] [PubMed] [Google Scholar]
- 8.Sebolt-Leopold J.S., Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat. Rev. Cancer. 2004;4:937–947. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- 9.Roskoski R. RAF protein-serine/threonine kinases: Structure and regulation. Biochem. Biophys. Res. Commun. 2010;399:313–317. doi: 10.1016/j.bbrc.2010.07.092. [DOI] [PubMed] [Google Scholar]
- 10.Davies H., Bignell G.R., Cox C., Stephens P., Edkins S., Clegg S., Teague J., Woffendin H., Garnett M.J., Bottomley W., et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 11.Mochizuki H., Kennedy K., Shapiro S.G., Breen M. Braf mutations in canine cancers. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0129534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Decker B., Parker H.G., Dhawan D., Kwon E.M., Karlins E., Davis B.W., Ramos-Vara J.A., Bonney P.L., McNiel E.A., Knapp D.W., et al. Homologous mutation to human BRAF V600E is common in naturally occurring canine bladder cancer-evidence for a relevant model system and urine-based diagnostic test. Mol. Cancer Res. 2015 doi: 10.1158/1541-7786.MCR-14-0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLaughlin C.C., Wu X.C., Jemal A., Martin H.J., Roche L.M., Chen V.W. Incidence of noncutaneous melanomas in the US. Cancer. 2005;103:1000–1007. doi: 10.1002/cncr.20866. [DOI] [PubMed] [Google Scholar]
- 14.Chang A.E., Karnell L.H., Menck H.R. The national cancer data base report on cutaneous and noncutaneous melanoma. Cancer. 1998;83:1664–1678. doi: 10.1002/(SICI)1097-0142(19981015)83:8<1664::AID-CNCR23>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 15.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2015. Ca-A Cancer J. Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 16.Garbe C., Leiter U. Melanoma epidemiology and trends. Clinics in Dermatology. 2009;27:3–9. doi: 10.1016/j.clindermatol.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Brose M.S., Volpe P., Feldman M., Kumar M., Rishi I., Gerrero R., Einhorn E., Herlyn M., Minna J., Nicholson A. BRAF and RAS mutations in human lung cancer and melanoma. Cancer. Res. 2002;62:6997–7000. [PubMed] [Google Scholar]
- 18.Tsao H., Goel V., Wu H., Yang G., Haluska F.G. Genetic interaction between NRAS and BRAF mutations and PTEN/MMAC1 inactivation in melanoma. J. Invest. Dermatol. 2004;122:337–341. doi: 10.1046/j.0022-202X.2004.22243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goel V.K., Lazar A.J., Warneke C.L., Redston M.S., Haluska F.G. Examination of mutations in BRAF, NRAS, and PTEN in primary cutaneous melanoma. J. Invest. Dermatol. 2006;126:154–160. doi: 10.1038/sj.jid.5700026. [DOI] [PubMed] [Google Scholar]
- 20.Simpson R.M., Bastian B.C., Michael H.T., Webster J.D., Prasad M.L., Conway C.M., Prieto V.M., Gary J.M., Goldschmidt M.H., Esplin D.G., et al. Sporadic naturally occurring melanoma in dogs as a preclinical model for human melanoma. Pigm. Cell Melanoma Res. 2014;27:37–47. doi: 10.1111/pcmr.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillard M., Cadieu E., De Brito C., Abadie J., Vergier B., Devauchelle P., Degorce F., Dreano S., Primot A., Dorso L., et al. Naturally occurring melanomas in dogs as models for non-uv pathways of human melanomas. Pigm. Cell Melanoma Res. 2014;27:90–102. doi: 10.1111/pcmr.12170. [DOI] [PubMed] [Google Scholar]
- 22.Shelly S., Chien M.B., Yip B., Kent M.S., Theon A.P., McCallan J.L., London C.A. Exon 15 BRAF mutations are uncommon in canine oral malignant melanomas. Mamm Genome. 2005;16:211–217. doi: 10.1007/s00335-004-2441-x. [DOI] [PubMed] [Google Scholar]
- 23.Fowles J., Denton C., Gustafson D. Comparative analysis of MAPK and PI3K/AKT pathway activation and inhibition in human and canine melanoma. Vet. Comp. Oncol. 2013 doi: 10.1111/vco.12044. [DOI] [PubMed] [Google Scholar]
- 24.Maldonado J.L., Fridlyand J., Patel H., Jain A.N., Busam K., Kageshita T., Ono T., Albertson D.G., Pinkel D., Bastian B.C. Determinants of BRAF mutations in primary melanomas. J. Nat. Cancer Inst. 2003;95:1878–1890. doi: 10.1093/jnci/djg123. [DOI] [PubMed] [Google Scholar]
- 25.Edwards R., Ward M., Wu H., Medina C., Brose M., Volpe P., Nussen-Lee S., Haupt H., Martin A., Herlyn M. Absence of BRAF mutations in uv-protected mucosal melanomas. J. Med. Genet. 2004;41:270–272. doi: 10.1136/jmg.2003.016667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pollock P.M., Harper U.L., Hansen K.S., Yudt L.M., Stark M., Robbins C.M., Moses T.Y., Hostetter G., Wagner U., Kakareka J., et al. High frequency of BRAF mutations in nevi. Nat. Genet. 2003;33:19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- 27.Knowles M.A., Hurst C.D. Molecular biology of bladder cancer: New insights into pathogenesis and clinical diversity. Nat. Rev. Cancer. 2015;15:25–41. doi: 10.1038/nrc3817. [DOI] [PubMed] [Google Scholar]
- 28.Boulalas L., Zaravinos A., Delakas D., Spandidos D.A. Mutational analysis of the BRAF gene in transitional cell carcinoma of the bladder. Int. J. Biol. Marker. 2009;24:17–21. doi: 10.1177/172460080902400103. [DOI] [PubMed] [Google Scholar]
- 29.Stoehr R., Brinkmann A., Filbeck T., Gamper C., Wild P., Blaszyk H., Hofstaedter F., Knuechel R., Hartmann A. No evidence for mutation of b-raf in urothelial carcinomas of the bladder and upper urinary tract. Oncol. Rep. 2004;11:137–141. doi: 10.3892/or.11.1.137. [DOI] [PubMed] [Google Scholar]
- 30.Jebar A.H., Hurst C.D., Tomlinson D.C., Johnston C., Taylor C.F., Knowles M.A. FGFR3 and RAS gene mutations are mutually exclusive genetic events in urothelial cell carcinoma. Oncogene. 2005;24:5218–5225. doi: 10.1038/sj.onc.1208705. [DOI] [PubMed] [Google Scholar]
- 31.Di Martino E., L’Hote C.G., Kennedy W., Tomlinson D.C., Knowles M.A. Mutant fibroblast growth factor receptor 3 induces intracellular signaling and cellular transformation in a cell type- and mutation-specific manner. Oncogene. 2009;28:4306–4316. doi: 10.1038/onc.2009.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knapp D.W., Ramos-Vara J.A., Moore G.E., Dhawan D., Bonney P.L., Young K.E. Urinary bladder cancer in dogs, a naturally occurring model for cancer biology and drug development. ILAR J. 2014;55:100–118. doi: 10.1093/ilar/ilu018. [DOI] [PubMed] [Google Scholar]
- 33.Knapp D.W., Glickman N.W., Denicola D.B., Bonney P.L., Lin T.L., Glickman L.T. Naturally-occurring canine transitional cell carcinoma of the urinary bladder a relevant model of human invasive bladder cancer. Urol. Oncol. 2000;5:47–59. doi: 10.1016/S1078-1439(99)00006-X. [DOI] [PubMed] [Google Scholar]
- 34.Kollermann J., Albrecht H., Schlomm T., Huland H., Graefen M., Bokemeyer C., Simon R., Sauter G., Wilczak W. Activating braf gene mutations are uncommon in hormone refractory prostate cancer in caucasian patients. Oncol. Lett. 2010;1:729–732. doi: 10.3892/ol_00000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu T., Willmore-Payne C., Layfield L.J., Holden J.A. Lack of BRAF activating mutations in prostate adenocarcinoma: A study of 93 cases. Appl. Immunohistochem. Mol. Morp. 2009;17:121–125. doi: 10.1097/PAI.0b013e31818816b9. [DOI] [PubMed] [Google Scholar]
- 36.Palmieri C., Lean F.Z., Akter S.H., Romussi S., Grieco V. A retrospective analysis of 111 canine prostatic samples: Histopathological findings and classification. Res. Vet. Sci. 2014;97:568–573. doi: 10.1016/j.rvsc.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 37.Cornell K.K., Bostwick D.G., Cooley D.M., Hall G., Harvey H.J., Hendrick M.J., Pauli B.U., Render J.A., Stoica G., Sweet D.C., et al. Clinical and pathologic aspects of spontaneous canine prostate carcinoma: A retrospective analysis of 76 cases. Prostate. 2000;45:173–183. doi: 10.1002/1097-0045(20001001)45:2<173::AID-PROS12>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 38.LeRoy B.E., Nadella M.V., Toribio R.E., Leav I., Rosol T.J. Canine prostate carcinomas express markers of urothelial and prostatic differentiation. Vet. Pathol. 2004;41:131–140. doi: 10.1354/vp.41-2-131. [DOI] [PubMed] [Google Scholar]
- 39.Sorenmo K.U., Goldschmidt M., Shofer F., Goldkamp C., Ferracone J. Immunohistochemical characterization of canine prostatic carcinoma and correlation with castration status and castration time. Vet.Comp. Oncol. 2003;1:48–56. doi: 10.1046/j.1476-5829.2003.00007.x. [DOI] [PubMed] [Google Scholar]
- 40.Louis D.N., Ohgaki H., Wiestler O.D., Cavenee W.K., Burger P.C., Jouvet A., Scheithauer B.W., Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta. Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schindler G., Capper D., Meyer J., Janzarik W., Omran H., Herold-Mende C., Schmieder K., Wesseling P., Mawrin C., Hasselblatt M. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta. Neuropathol. 2011;121:397–405. doi: 10.1007/s00401-011-0802-6. [DOI] [PubMed] [Google Scholar]
- 42.Korshunov A., Meyer J., Capper D., Christians A., Remke M., Witt H., Pfister S., von Deimling A., Hartmann C. Combined molecular analysis of BRAF and IDH1 distinguishes pilocytic astrocytoma from diffuse astrocytoma. Acta. Neuropathol. 2009;118:401–405. doi: 10.1007/s00401-009-0550-z. [DOI] [PubMed] [Google Scholar]
- 43.Pfister S., Janzarik W.G., Remke M., Ernst A., Werft W., Becker N., Toedt G., Wittmann A., Kratz C., Olbrich H. BRAF gene duplication constitutes a mechanism of mapk pathway activation in low-grade astrocytomas. J. Clin.Invest. 2008;118:1739. doi: 10.1172/JCI33656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones D.T., Kocialkowski S., Liu L., Pearson D.M., Bäcklund L.M., Ichimura K., Collins V.P. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008;68:8673–8677. doi: 10.1158/0008-5472.CAN-08-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kieran M.W. Targeting BRAF in pediatric brain tumors. [(accessed on 20 August 2015)]. Available online: http://europepmc.org/abstract/med/24857135.
- 46.Dasgupta T., Haas-Kogan D.A. The combination of novel targeted molecular agents and radiation in the treatment of pediatric gliomas. Front. Oncol. 2013;3 doi: 10.3389/fonc.2013.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas R., Duke S.E., Wang H.J., Breen T.E., Higgins R.J., Linder K.E., Ellis P., Langford C.F., Dickinson P.J., Olby N.J., et al. 'Putting our heads together': Insights into genomic conservation between human and canine intracranial tumors. J. Neuro-Oncol. 2009;94:333–349. doi: 10.1007/s11060-009-9877-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tiacci E., Trifonov V., Schiavoni G., Holmes A., Kern W., Martelli M.P., Pucciarini A., Bigerna B., Pacini R., Wells V.A., et al. BRAF mutations in hairy-cell leukemia. N. Engl. J. Med. 2011;364:2305–2315. doi: 10.1056/NEJMoa1014209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee J., Yoo N., Soung Y., Kim H., Park W., Kim S., Lee J., Park J., Cho Y., Kim C. BRAF mutations in non-hodgkin’s lymphoma. Brit. J. Cancer. 2003;89:1958–1960. doi: 10.1038/sj.bjc.6601371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bonello L., Voena C., Ladetto M., Boccadoro M., Palestro G., Inghirami G., Chiarle R. BRAF gene is not mutated in plasma cell leukemia and multiple myeloma. Leukemia. 2003;17:2238–2240. doi: 10.1038/sj.leu.2403116. [DOI] [PubMed] [Google Scholar]
- 51.Lee J., Soung Y., Park W., Kim S., Nam S., Min W., Lee J., Yoo N., Lee S. BRAF mutations in acute leukemias. Leukemia. 2004;18:170–172. doi: 10.1038/sj.leu.2403201. [DOI] [PubMed] [Google Scholar]
- 52.Badalian-Very G., Vergilio J.-A., Degar B.A., MacConaill L.E., Brandner B., Calicchio M.L., Kuo F.C., Ligon A.H., Stevenson K.E., Kehoe S.M. Recurrent BRAF mutations in langerhans cell histiocytosis. Blood. 2010;116:1919–1923. doi: 10.1182/blood-2010-04-279083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suter S.E., Small G.W., Seiser E.L., Thomas R., Breen M., Richards K.L. FLT3 mutations in canine acute lymphocytic leukemia. BMC Cancer. 2011;11 doi: 10.1186/1471-2407-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Breen M., Modiano J.F. Evolutionarily conserved cytogenetic changes in hematological malignancies of dogs and humans-man and his best friend share more than companionship. Chromosome Res. 2008;16:145–154. doi: 10.1007/s10577-007-1212-4. [DOI] [PubMed] [Google Scholar]
- 55.Ma Y., Longley B.J., Wang X., Blount J.L., Langley K., Caughey G.H. Clustering of activating mutations in c-kit’s juxtamembrane coding region in canine mast cell neoplasms. J. Invest. Dermatol. 1999;112:165–170. doi: 10.1046/j.1523-1747.1999.00488.x. [DOI] [PubMed] [Google Scholar]
- 56.London C.A., Galli S.J., Yuuki T., Hu Z.Q., Helfand S.C., Geissler E.N. Spontaneous canine mast cell tumors express tandem duplications in the proto-oncogene c-kit. Exp. Hematol. 1999;27:689–697. doi: 10.1016/S0301-472X(98)00075-7. [DOI] [PubMed] [Google Scholar]
- 57.Nikiforov Y.E., Nikiforova M.N. Molecular genetics and diagnosis of thyroid cancer. Nat. Rev. Endocrinol. 2011;7:569–580. doi: 10.1038/nrendo.2011.142. [DOI] [PubMed] [Google Scholar]
- 58.Xing M. BRAF mutation in thyroid cancer. Endocr-Related Cancer. 2005;12:245–262. doi: 10.1677/erc.1.0978. [DOI] [PubMed] [Google Scholar]
- 59.Wucherer K.L., Wilke V. Thyroid cancer in dogs: An update based on 638 cases (1995–2005) J. Amer. Anim. Hosp. Assn. 2010;46:249–254. doi: 10.5326/0460249. [DOI] [PubMed] [Google Scholar]
- 60.Ramos-Vara J.A., Miller M.A., Johnson G.C., Pace L.W. Immunohistochemical detection of thyroid transcription factor-1, thyroglobulin, and calcitonin in canine normal, hyperplastic, and neoplastic thyroid gland. Vet. Pathol. 2002;39:480–487. doi: 10.1354/vp.39-4-480. [DOI] [PubMed] [Google Scholar]
- 61.Cardarella S., Ogino A., Nishino M., Butaney M., Shen J., Lydon C., Yeap B.Y., Sholl L.M., Johnson B.E., Jänne P.A. Clinical, pathologic, and biologic features associated with BRAF mutations in non–small cell lung cancer. Clin. Cancer Res. 2013;19:4532–4540. doi: 10.1158/1078-0432.CCR-13-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marchetti A., Felicioni L., Malatesta S., Sciarrotta M.G., Guetti L., Chella A., Viola P., Pullara C., Mucilli F., Buttitta F. Clinical features and outcome of patients with non–small-cell lung cancer harboring BRAF mutations. J. Clin. Oncol. 2011;29:3574–3579. doi: 10.1200/JCO.2011.35.9638. [DOI] [PubMed] [Google Scholar]
- 63.Hegedüs L. The thyroid nodule. N.Engl.J. Med. 2004;351:1764–1771. doi: 10.1056/NEJMcp031436. [DOI] [PubMed] [Google Scholar]
- 64.Nikiforov Y.E., Steward D.L., Robinson-Smith T.M., Haugen B.R., Klopper J.P., Zhu Z., Fagin J.A., Falciglia M., Weber K., Nikiforova M.N. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J. Clin. Endocrinol. Metab. 2009;94:2092–2098. doi: 10.1210/jc.2009-0247. [DOI] [PubMed] [Google Scholar]
- 65.Crowley E., Di Nicolantonio F., Loupakis F., Bardelli A. Liquid biopsy: Monitoring cancer-genetics in the blood. Nat. Rev. Clin. Oncol. 2013;10:472–484. doi: 10.1038/nrclinonc.2013.110. [DOI] [PubMed] [Google Scholar]
- 66.Sanmamed M.F., Fernandez-Landazuri S., Rodriguez C., Zarate R., Lozano M.D., Zubiri L., Perez-Gracia J.L., Martin-Algarra S., Gonzalez A. Quantitative cell-free circulating BRAFV600E mutation analysis by use of droplet digital PCR in the follow-up of patients with melanoma being treated with BRAF inhibitors. Clin. Chem. 2015;61:297–304. doi: 10.1373/clinchem.2014.230235. [DOI] [PubMed] [Google Scholar]
- 67.Shinozaki M., O'Day S.J., Kitago M., Amersi F., Kuo C., Kim J., Wang H.J., Hoon D.S. Utility of circulating B-RAF DNA mutation in serum for monitoring melanoma patients receiving biochemotherapy. Clin. Cancer Res. 2007;13:2068–2074. doi: 10.1158/1078-0432.CCR-06-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bell F.W., Klausner J.S., Hayden D.W., Feeney D.A., Johnston S.D. Clinical and pathologic features of prostatic adenocarcinoma in sexually intact and castrated dogs: 31 cases (1970–1987) J. Amer. Vet. Med. Assoc. 1991;199:1623–1630. [PubMed] [Google Scholar]
- 69.Childress M.O., Adams L.G., Ramos-Vara J.A., Freeman L.J., He S., Constable P.D., Knapp D.W. Results of biopsy via transurethral cystoscopy and cystotomy for diagnosis of transitional cell carcinoma of the urinary bladder and urethra in dogs: 92 cases (2003–2008) J. Amer. Vet. Med. Assoc. 2011;239:350–356. doi: 10.2460/javma.239.3.350. [DOI] [PubMed] [Google Scholar]
- 70.Shapiro S.G., Raghunath S., Williams C., Motsinger-Reif A.A., Cullen J.M., Liu T., Albertson D., Ruvolo M., Bergstrom Lucas A., Jin J., et al. Canine urothelial carcinoma: Genomically aberrant and comparatively relevant. Chromosome Res. 2015;23:311–331. doi: 10.1007/s10577-015-9471-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Powe J.R., Canfield P.J., Martin P.A. Evaluation of the cytologic diagnosis of canine prostatic disorders. Vet. Clin .Path. 2004;33:150–154. doi: 10.1111/j.1939-165X.2004.tb00365.x. [DOI] [PubMed] [Google Scholar]
- 72.Smith J. Canine prostatic disease: A review of anatomy, pathology, diagnosis, and treatment. Theriogenology. 2008;70:375–383. doi: 10.1016/j.theriogenology.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 73.Huggett J.F., Novak T., Garson J.A., Green C., Morris-Jones S.D., Miller R.F., Zumla A. Differential susceptibility of pcr reactions to inhibitors: An important and unrecognised phenomenon. BMC Rese.Notes. 2008;1 doi: 10.1186/1756-0500-1-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Toye B., Woods W., Bobrowska M., Ramotar K. Inhibition of pcr in genital and urine specimens submitted for chlamydia trachomatis testing. J. Clin. Microbiol. 1998;36:2356–2358. doi: 10.1128/jcm.36.8.2356-2358.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huggett J.F., Whale A. Digital pcr as a novel technology and its potential implications for molecular diagnostics. Clin.Chem. 2013;59:1691–1693. doi: 10.1373/clinchem.2013.214742. [DOI] [PubMed] [Google Scholar]
- 76.Balch C.M., Gershenwald J.E., Soong S.J., Thompson J.F., Atkins M.B., Byrd D.R., Buzaid A.C., Cochran A.J., Coit D.G., Ding S., et al. Final version of 2009 AJCC melanoma staging and classification. J. Clin.Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chapman P.B., Hauschild A., Robert C., Haanen J.B., Ascierto P., Larkin J., Dummer R., Garbe C., Testori A., Maio M. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Eng. J. Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Robert C., Karaszewska B., Schachter J., Rutkowski P., Mackiewicz A., Stroiakovski D., Lichinitser M., Dummer R., Grange F., Mortier L. Improved overall survival in melanoma with combined dabrafenib and trametinib. N. Eng. J. Med. 2015;372:30–39. doi: 10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- 79.Hauschild A., Grob J.-J., Demidov L.V., Jouary T., Gutzmer R., Millward M., Rutkowski P., Blank C.U., Miller W.H., Kaempgen E. Dabrafenib in BRAF-mutated metastatic melanoma: A multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 80.Follows G.A., Sims H., Bloxham D.M., Zenz T., Hopper M.A., Liu H., Bench A., Wright P., van't Veer M.B., Scott M.A. Rapid response of biallelic BRAF V600E mutated hairy cell leukaemia to low dose vemurafenib. Brit. J. Haematol. 2013;161:150–153. doi: 10.1111/bjh.12201. [DOI] [PubMed] [Google Scholar]
- 81.Haroche J., Cohen-Aubart F., Emile J.-F., Arnaud L., Maksud P., Charlotte F., Cluzel P., Drier A., Hervier B., Benameur N. Dramatic efficacy of vemurafenib in both multisystemic and refractory erdheim-chester disease and langerhans cell histiocytosis harboring the BRAF V600E mutation. Blood. 2013;121:1495–1500. doi: 10.1182/blood-2012-07-446286. [DOI] [PubMed] [Google Scholar]
- 82.Munoz J., Schlette E., Kurzrock R. Rapid response to vemurafenib in a heavily pretreated patient with hairy cell leukemia and a BRAF mutation. J. Clin.Oncol. 2013;31:e351–e352. doi: 10.1200/JCO.2012.45.7739. [DOI] [PubMed] [Google Scholar]
- 83.Holderfield M., Deuker M.M., McCormick F., McMahon M. Targeting RAF kinases for cancer therapy: BRAF-mutated melanoma and beyond. Nat. Rev. Cancer. 2014;14:455–467. doi: 10.1038/nrc3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kopetz S., Desai J., Chan E., Hecht J., O'dwyer P., Lee R., Nolop K., Saltz L. PLX4032 in metastatic colorectal cancer patients with mutant BRAF tumors; Proceedings of ASCO Annual Meeting Proceedings; Chicago, USA. 4–8 June 2010; p. 3534. [Google Scholar]
- 85.Prahallad A., Sun C., Huang S., di Nicolantonio F., Salazar R., Zecchin D., Beijersbergen R.L., Bardelli A., Bernards R. Unresponsiveness of colon cancer to BRAF (V600E) inhibition through feedback activation of EGFR. Nature. 2012;483:100–103. doi: 10.1038/nature10868. [DOI] [PubMed] [Google Scholar]
- 86.Montero-Conde C., Ruiz-Llorente S., Dominguez J.M., Knauf J.A., Viale A., Sherman E.J., Ryder M., Ghossein R.A., Rosen N., Fagin J.A. Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF-mutant thyroid carcinomas. Cancer Disc. 2013;3:520–533. doi: 10.1158/2159-8290.CD-12-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]