Abstract
Background
This study aimed to investigate the relationship between obesity and chronic laryngitis in South Korea using data from the Korea National Health and Nutrition Examination Surveys (KNHANES) collected during 2008-2010.
Methods
KNHANES was a cross-sectional survey of the civilian, non-institutionalized population of South Korea (n = 13,819). Obesity status was measured by using BMI and waist circumference.
Results
Among the population over 19 years of age, the prevalence of chronic laryngitis was 4.0 ± 0.4%. Chronic laryngitis was significantly associated with age, BMI, waist circumference, fat proportion, both systolic and diastolic blood pressure, fasting blood sugar, triglycerides, and high-density lipoprotein cholesterol in women. Old age and current smoking were significantly associated with chronic laryngitis in men. Obese women were at a higher risk for chronic laryngitis than women without obesity (odds ratio (OR) 2.022, 95% confidence interval (95% CI) 1.412-2.895) after further adjustment for confounders. Women with abdominal obesity were also at higher risk for chronic laryngitis (OR 1.475, 95% CI 1.024-2.126).
Conclusion
Obese women in Korea have an elevated risk for developing chronic laryngitis. Further epidemiological and experimental studies are necessary to clarify the impact of obesity on this condition.
Key Words: Laryngitis, Obesity, Epidemiology
Introduction
Obesity has increased dramatically in both developing and developed countries [1]. Global mortality due to overweight and obesity is approximately 2.8 million annually [2]. In 2008, 10 and 14% of the global population of men and women, respectively, were obese, with a BMI ≥ 30 kg/m2; in 1980, only 5% of men and 8% of women could be classified as obese [2]. Therefore, global prevalence of obesity has doubled between 1980 and 2008 [2]. According to the World Health Organization (WHO), 35% of adults worldwide over 20 years of age are overweight, defined as having a BMI ≥ 25 kg/m2 (34% for men and 35% for women) [2]. In each geographical region covered by the WHO survey, women were more likely to be obese than men [2]. For example, in Africa, the eastern Mediterranean, and Southeast Asia, the prevalence of obesity among women was roughly twice that of men [2].
Chronic laryngitis (CL) has been described as a chronic inflammation of the larynx, which typically develops gradually and whose underlying signs and symptoms can wax and wane over long periods of time [3]. When the larynx is not functioning properly, individuals experience voice problems as well as difficulty aspirating into the trachea, resulting in breathing disturbances. Repeated chronic inflammation of the laryngeal epithelium can contribute to the formation of precancerous lesions, potentially leading to laryngeal cancer [4].
The strong association between obesity and diabetes mellitus, ischemic heart disease, hypertension, hyperlipidemia or certain cancers is well-known [5]. Meanwhile, obesity is associated with a significant 1.5- to 2-fold increase in the risk of gastroesophageal reflux disease (GERD) symptoms [6]. GERD affects the larynx and causes many patients to develop reflux laryngitis or laryngopharyngeal reflux (LPR) [7]. LPR can be thought to lead to CL [8]. We hypothesized that obesity might play an important role in CL. However, the relationship between obesity and CL has not been recognized. Here, we used data from the Korea National Health and Nutrition Examination Survey (KNHANES) to study the factors associated with CL. The KNHANES is a national surveillance system that has been assessing the health and nutritional status of Koreans since 1998. Based on the National Health Promotion Act, the surveys have been conducted by the Korea Centers for Disease Control and Prevention [9].
To our knowledge, there have been no nationwide data studies of CL diagnosed by otolaryngologists, or studies of CL-associated factors in Asia. Therefore, we intended to investigate whether CL is associated with certain risk factors by using the large representative population sample from KNHANES.
Participants and Methods
Study Population
This study was based on data collected during the 2008-2010 KNHANES. In conducting KNHANES, a field survey team that included an otolaryngologist, an ophthalmologist, and nurse examiners moved with a mobile examination unit and performed interviews and physical examinations. The survey consisted of a health interview, a nutritional survey, and a health examination survey. The survey amasses data via household interviews and by direct standardized physical examinations conducted in specially equipped mobile examination centers. The KNHANES methodology was previously described in detail [10,11,12].
The sample included 14,070 participants over the age of 19 years. We excluded 251 subjects suffering from various cancers, chronic kidney disease, chronic liver disease, and active infectious diseases such as active tuberculosis. Ultimately, the data of 13,819 subjects were used in the analyses. Written informed consent was obtained from all participants prior to the survey, and approval for this study was obtained from the Institutional Review Board of the Catholic University of Korea in Seoul, South Korea.
Survey for CL
Participants who were 19 years of age or older were examined. Laryngologic interviews and vocal fold examinations were performed using a 4-mm 70° angled rigid endoscope with a CCD camera. The Epidemiologic Survey Committee of the Korean Otolaryngologic Society prepared a CL diagnostic protocol. The laryngologic questionnaire asked whether the participants have had problems with their voice. The participants with a positive response were considered as suffering from CL. Participants who responded positively to this question were then queried concerning the duration by the following questions: ‘How long is this voice problem?’ (>3 weeks or <3 weeks). Laryngoscopic findings of laryngitis and/or inflammation, including Reinke's edema, pseudosulcus, erythema, edema, or thick endolaryngeal mucus were diagnosed as CL. The Epidemiologic Survey Committee of the Korean Otolaryngologic Society verified the quality of the survey, which was conducted by periodically visiting the mobile examination units, educating the participating residents, obtaining the laryngeal examination data, and data-proofing using video documentation of the larynx throughout the study. Two otolaryngologic surgeons from the Korean Otolaryngologic Society subsequently verified the video documentation and assessed the disease decision protocol. Documentation of the video was obtained as 640 × 480-sized Audio Video Interleave files, which were compressed by DivX 4.12 codec using a compression rate of 6 Mb/s.
Lifestyle Habits
Medical history and lifestyle habits were collected using self-reported questionnaires. Smoking history was categorized into the three groups: current smoker, ex-smoker, and nonsmoker. Subjects who drank more than 30 g/day of alcohol were designated as drinkers. Regular exercise was defined as strenuous physical activity performed for a minimum of 20 min, three times a week.
Anthropometric and Laboratory Measurements
Weight and height were measured by well-trained medical professionals. Standing height was measured with the subject facing directly ahead with their shoes off, feet together, arms at the sides, and heels, buttocks, and upper back in contact with the wall. The unit of height was measured in centimeters to the nearest decimal point using SECA 225 (SECA, Hamburg, Germany). Waist circumference (WC) was measured at the level of the midpoint between the iliac crest and the costal margin at the end of a normal expiration to the nearest 0.1 cm. Weight was measured using a GL-6000-20 scale (CasKorea, Seoul, South Korea) in kilograms to the nearest decimal point. BMI was calculated as weight (kg)/height (m2). Based on BMI, we defined general obesity as a BMI ≥ 25 kg/m2 and general overweight as a BMI between 23 Kg/ m2 and 25 kg/m2 [3,13]. The cutoff points for abdominal obesity were defined as a WC ≥ 90 cm for men and ≥ 85 cm for women [14].
Dual energy X-ray absorptiometry (Hologic Discovery-W; Hologic, Bedford, MA, USA) was used to measure participants’ body compositions. Assuming that all non-fat and non-bone tissues were skeletal muscles, appendicular muscle mass (ASM) was calculated as the sum of muscle mass in arms and legs. The fat proportion was defined as the proportion of total fat mass divided by body weight. According to the definitions proposed in previous studies, we used muscle mass indices as ASM divided by weight (ASM/Wt, %). Blood pressure (BP) was measured while subjects were in a sitting position following a 5-min rest period. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured on the right arm using a mercury sphygmomanometer (Baumanometer, W. A. Baum Co., Copiague, NY, USA). To assess serum levels of biochemical markers, blood samples were obtained from the antecubital veins of the subjects following a 10- to 12-hour overnight, fast. Serum levels of fasting blood sugar (FBS), total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL) cholesterol, and low-density lipoprotein (LDL) cholesterol were measured using an enzymatic method (Hitachi Automatic Analyzer 7600, Hitachi, Tokyo, Japan).
Statistical Analysis
Statistical analyses were performed using the Statistical Analysis Software (SAS) survey procedure (v. 9.3; SAS Institute, Cary, NC, USA) to reflect the complex sampling design and sampling weights of KNHANES and to provide nationally representative prevalence estimates. The procedures included unequal probabilities of selection, oversampling, and nonresponse so that inferences could be made about the Korean adolescent participants.
The prevalence and 95% confidence intervals (95% CI) for chronic laryngitis was calculated. In the univariate analysis, the Rao-Scott chi-square test (using PROC SURVEYFREQ in SAS) and logistic regression analysis (using PROC SURVEYLOGISTIC in SAS) were used to test the association between CL and risk factors in a complex sampling design. Participants’ characteristics were described using means and standard errors (SE) for continuous variables, and numbers and percentages for categorical variables. Simple and multiple linear regression analyses were used to examine the association between CL and obesity.
We did not adjust (model 1), first adjusted for age (model 2), and then adjusted for the variables in model 2 plus smoking status, alcohol intake, and physical activity (model 3). P values were two-tailed, and a p < 0.05 was considered significant.
Results
Among the 13,819 participants aged 19 years or over, 549 had experienced CL; the prevalence of CL was 4.0 ± 0.4%. The baseline characteristics of the study subjects according to CL are shown in table 1. The mean age of those with CL was significantly higher than those without CL for both sexes. Current smoking and high SBP were significantly associated with CL in men. Mean values of WC, BMI, fat proportion, SBP, DBP, FBS, and TG were significantly higher among women with CL than among women without CL. However, the mean HDL cholesterol level was lower in women with CL than in women without CL.
Table 1.
Analysis of factors potentially associated with chronic laryngitis (n = 13,819)
| Parameter | Male |
Female |
||||||
|---|---|---|---|---|---|---|---|---|
| yes (n = 299) | no (n = 5,649) | p value | yes (n = 250) | no (n = 7,621) | p value | |||
| Age, years | 48.6 ± 1.1 | 42.9 ± 0.3 | <0.0001* | 50.9 ± 1.5 | 45.5 ± 0.3 | 0.0001* | ||
| Current smoker, % | 6.1 ± 0.8 | 4.1 ± 0.5 | 0.0087* | 3.7 ± 1.1 | 2.9 ± 0.4 | 0.4249 | ||
| Heavy drinker, % | 6.0 ± 1.0 | 4.8 ± 0.5 | 0.1585 | 2.8 ± 1.3 | 3.0 ± 0.4 | 0.8348 | ||
| Routine exercise, % | 4.5 ± 0.6 | 5.2 ± 0.6 | 0.3280 | 3.4 ± 0.7 | 2.9 ± 0.4 | 0.4232 | ||
| Waist circumference, cm | 84.7 ± 0.6 | 83.7 ± 0.2 | 0.1500 | 80.5 ± 1.0 | 77.4 ± 0.2 | 0.0030* | ||
| BMI, kg/m2 | 24.1 ± 0.2 | 24.0 ± 0.1 | 0.5588 | 24.2 ± 0.3 | 23.1 ± 0.1 | <0.0001* | ||
| Fat proportion, % | 22.0 ± 0.4 | 21.9 ± 0.1 | 0.7373 | 33.7 ± 0.4 | 32.8 ± 0.1 | 0.0404* | ||
| ASM/Wt, % | 32.1 ± 0.2 | 32.5 ± 0.5 | 0.4670 | 25.2 ± 0.2 | 25.5 ± 0.1 | 0.5160 | ||
| Systolic BP, mm Hg | 122.5 ± 1.2 | 120.0 ± 0.3 | 0.0333* | 120.0 ± 1.6 | 115.1 ± 0.3 | 0.0031* | ||
| Diastolic BP, mm Hg | 80.4 ± 0.8 | 79.8 ± 0.2 | 0.4481 | 76.7 ± 1.0 | 73.8 ± 0.2 | 0.0025* | ||
| Glucose, mg/dl | 100.5 ± 1.8 | 97.9 ± 0.4 | 0.1719 | 98.1 ± 1.4 | 94.6 ± 0.3 | 0.0150* | ||
| Total cholesterol, mg/dl | 185.4 ± 2.6 | 186.4 ± 0.6 | 0.7141 | 187.8 ± 2.9 | 185.8 ± 0.6 | 0.4843 | ||
| HDL cholesterol, mg/dl | 49.8 ± 1.0 | 49.6 ± 0.2 | 0.8827 | 52.5 ± 1.0 | 55.5 ± 0.2 | 0.0039* | ||
| LDL cholesterol, mg/dl | 107.3 ± 2.8 | 107.7 ± 0.6 | 0.9092 | 109.9 ± 2.6 | 108.7 ± 0.5 | 0.6323 | ||
| Triglyceride, mg/dl | 130 | 125.7 | 0.4558 | 105.1 | 91.9 | 0.0245* | ||
| (119.7–141.2) | (122.8–128.6) | (93.6–118.0) | (90.4–93.4) | |||||
ASM = Appendicular skeletal muscle; Wt = weight; BP = blood pressure; HDL = high-density lipoprotein; LDL = low-density lipoprotein.
Plus-minus values are mean ± SE or % ± SE.
Indicates p value < 0.05.
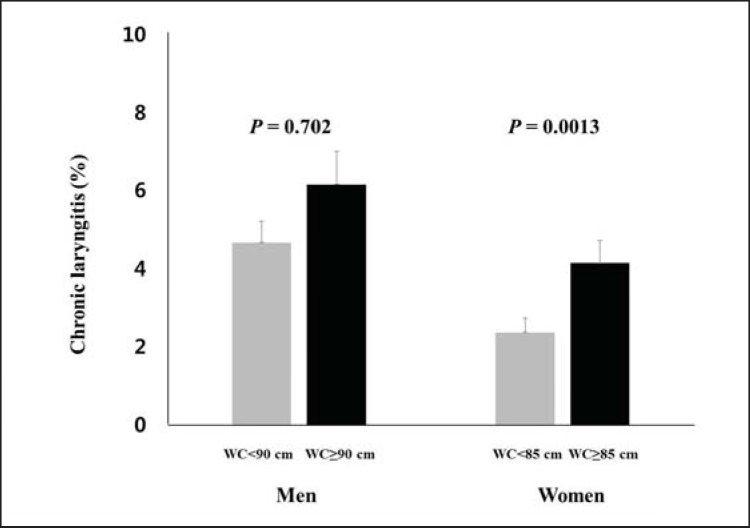
Table 2 shows the risk of developing CL according to obesity status in both sexes after adjustment for confounders. The adjusted odds ratios (ORs) for CL are not significant in generally obese and abdominally obese men (fig. 1 and 2).
Table 2.
Logistic regression models of obesity for chronic laryngitis
| Parameter | Odds ratio (95% CI) |
|||||
|---|---|---|---|---|---|---|
| Male |
Female |
|||||
| model 1 | model 2 | model 3 | model 1 | model 2 | model 3 | |
| Overweight | 0.909 (0.617–1.339) |
0.892 (0.607–1.312) |
0.915 (0.618–1.354) |
1.373 (0.899–2.095) |
1.213 (0.803–1.830) |
1.166 (0.763–1.782) |
|
| ||||||
| Obesity | 1.180 (0.856–1.628) |
1.197 (0.865–1.655) |
1.251 (0.899–1.740) |
2.259 (1.567–3.257) |
1.939 (1.352–2.780) |
2.022 (1.412–2.895) |
|
| ||||||
| P for trend | 0.3240 | 0.2912 | 0.1949 | <0.0001 | 0.0004 | 0.0002 |
|
| ||||||
| Waist circumference | 1.340 (0.976–1.840) |
1.232 (0.894–1.697) |
1.238 (0.900–1.702) |
1.781 (1.249–2.540) |
1.461 (1.020–2.091) |
1.475 (1.024–2.126) |
|
| ||||||
| P for trend | 0.0706 | 0.2026 | 0.1890 | 0.0014 | 0.0387 | 0.0369 |
Model 1: Unadjusted. Model 2: Adjusted for age. Model 3: Adjusted for age, smoking status, alcohol intake, regular exercise.
Fig. 1.
Prevalence of CL based on the BMI of each sex. Values are represented as mean ± SE.
Fig. 2.
Prevalence of CL based on WC of each sex. Values are represented as mean ± SE.
Meanwhile, in women with normal BMI, the risk for having CL was significantly associated with overweight status (OR (95% CI) 1.373 (0.899-2.095)) and obesity (OR (95% CI) 2.259 (1.567-3.257)) after adjusting for all the confounders in model 1. Additionally, obese women had a higher risk of CL than women without obesity, and this relationship persisted even after further adjustment (OR (95% CI) 1.939 (1.352-2.780) in model 2 and 2.022 (1.412-2.895) in model 3). Women with abdominal obesity were also at a higher risk for CL in all models (OR (95% CI) 1.781 (1.249-2.540) in model 1, 1.461 (1.020-2.091) in model 2, and 1.475 (1.024-2.126) in model 3). Also, we analyzed using BMI and WC as continuous variables (table 3).
Table 3.
Logistic regression models of BMI for chronic laryngitis
| Parameter | Odds ratio (95% CI) |
|||||
|---|---|---|---|---|---|---|
| Male |
Female |
|||||
| model 1 | model 2 | model 3 | model 1 | model 2 | model 3 | |
| BMI | 1.013 | 1.02 | 1.026 | 1.087 | 1.072 | 1.074 |
| (0.971–1.056) | (0.976–1.066) | (0.981–1.072) | (1.048–1.128) | (1.032–1.114) | (1.033–1.117) | |
|
| ||||||
| P for trend | 0.5546 | 0.375 | 0.2616 | <0.0001 | 0.0004 | 0.0003 |
|
| ||||||
| Waist circumference | 1.011 | 1.006 | 1.006 | 1.03 | 1.02 | 1.021 |
| (0.996–1.027) | (0.991–1.022) | (0.991–1.022) | (1.011–1.049) | (1.001–1.04) | (1.001–1.041) | |
|
| ||||||
| P for trend | 0.1425 | 0.4412 | 0.4293 | 0.0016 | 0.0401 | 0.0352 |
Model 1: Unadjusted. Model 2: Adjusted for age. Model 3: Adjusted for age, smoking status, alcohol intake, regular exercise.
Discussion
This is the first nationwide study examining CL-associated factors in South Korea. This study showed that, compared with males, overweight and obese females have a higher risk for developing CL. In comparison to the non-CL population, the CL subjects were older. In males, we found CL to be associated with current smoker status and SBP. In females, CL was associated with WC, BMI, fat proportion, SBP, DBP, glucose, reduced HDL cholesterol, and increased TG.
A previous study [3] showed that CL had an incidence of 3.5 new cases per 1,000 people annually. Thus, 21% of individuals will have CL in their lifetime. CL is most commonly caused by laryngopharyngeal reflux, particularly in the USA, but it is also associated with other causes including poor laryngeal hygiene (smoking, excessive alcohol or caffeine intake), some bacterial and fungal infections (blastomycosis and tuberculosis), and more obscure conditions, such as the CL seen in glassblowers [15,16,17]. CL signs and symptoms associated with GERD are often referred to as LPR or reflux laryngitis. LPR is an extra-esophageal variant of GERD, because the main symptomatic region involves the laryngopharynx [18,19]. Approximately 10% of all otolaryngology patients and 50% of patients with voice complaints have been diagnosed with LPR [20]. Injury may occur as a result of reflux of gastroduodenal contents directly injuring the laryngeal mucosa, occurring chronically or even only once. Since a smaller amount of acid is required to injure the larynx as compared to the esophagus, it is believed that intermittent exposure to small amounts of gastric content can result in laryngitis. The diagnosis of LPR is usually made on the basis of presenting symptoms and associated laryngeal signs, including laryngeal edema and erythema [7]. Most subjects in the CL group had multiple symptoms, but dysphonia was the most common complaint which was seen in 53% of individuals, followed by pain in 45% and a globus sensation in 40% [3]. Laryngeal examinations almost always reveal diffuse but variably severe supraglottal and glottal erythema and edema. Excessive and sticky-thick mucus secretions may be observed in the valleculae, piriform sinuses, and endolarynx.
Age can affect esophageal function and CL. Oropharyngeal function changes with age, which may explain the age-related pattern of CL in our body. With age, the driving force of the tongue decreases, as does the amplitude of pharyngeal wall contraction, and there is also a reduction in pharyngeal swallowing, leading to food retention in the valleculae and piriform sinuses [21]. The afferent arm of the laryngo-upper-esophageal sphincter reflex is impaired, and the gag reflex is reportedly absent in 40% of healthy elderly individuals [21]. Age-related changes can also give rise to abnormal function in lower esophageal sphincter (LES) pressure, decrease LES length, and impair esophageal motility [21,22]. Abnormal esophageal function and LES could cause the retention of food and regurgitation of gastric juice, tending to damage the larynx [21,22].
Estrogen may be the cause of the gender-based differences we observed in CL prevalence. Elevated estrogen levels cause gastric acid secretion and GERD in women [23,24,25]. Obese women have a stronger correlation than do obese men [23,24,25].
Obesity can also play a major role in CL. Excess body weight with high abdominal circumference produces higher intra-abdominal pressure, reducing LES pressure [26,27]. Obesity also increases the prevalence of esophageal motility disorders [28]. In one study, esophageal transit time was significantly prolonged in obese subjects compared to lean subjects [28]. The increased esophageal transit time is considered to be a consequence of increased gastric and gastroesophageal junction resistance [28].
High TG levels were also a risk factor associated with CL in this study. Several studies have revealed that a high level of serum TG is an important predictive factor for GERD or erosive esophagitis among the components of metabolic syndrome [29,30,31].
Another CL-associated risk factor is smoking. Smoking weakens the pharyngeal reflexes such as the pharyngoglottal closure reflex and pharyngo-upper-esophageal contractile reflex [32,33]. Smoking also delays gastric emptying, thereby decreasing LES pressure, and impairing both esophageal acid clearance and reflexive pharyngeal swallowing [32,33]. Therefore, smoking may increase the gastric acid content of the lower esophagus, resulting in regurgitation into the pharynx. However, it is uncertain whether smoking is significantly associated with CL in men [32,33].
Hypertension was also a risk factor associated with CL in this study. Hypertension was found to be associated with GERD, after adjusting for BMI. In a Japanese study, hypertension was one of the independent risk factors for erosive esophagitis [34]. In Korea, calcium antagonists are frequently used antihypertensive drugs, which are able to decrease LES pressure. Thus, one limitation to our finding is that we did not investigate drug therapy. Another limitation of the present study is that it was a cross-sectional study; therefore, causal inferences could not be drawn. However, the study sample used was the representative sample of the South Korean population.
The major strength of this study was many covariates to be adjusted for. Also, this data was measured by checking the otolaryngologist, and we present the first relationship between obesity and CL.
There were no previous estimates of the factors associated with CL in the representative general population. In this study, data was based on a representative government-sponsored survey in South Korea. This study showed that the associated factors of CL in the KNHANES differed according to sex, and that in women CL is associated with obesity after adjusting for other confounding factors. Public acknowledgement and intervention measures to address CL risk factors are required to both prevent and manage the condition. Further studies are also needed to elucidate potential mechanisms underlying these relationships.
Disclosure Statement
The authors declare no conflict of interest.
Acknowledgments
We thank the 150 residents of the otorhinolaryngology departments of the 47 training hospitals in South Korea and members of the Division of Chronic Disease Surveillance in the Korea Centers for Disease Control & Prevention for participating in this survey and the dedicated work they provided. We are grateful to Jeong-A Kim, Mi-Ran Jang for valuable review this paper.
References
- 1.Haidar YM, Cosman BC. Obesity epidemiology. Clinics in colon and rectal surgery. 2011;24:205. doi: 10.1055/s-0031-1295684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . Global health observatory (GHO): Obesity 2008. Geneva: World Health Organization; 2014. [Google Scholar]
- 3.Stein DJ, Noordzij JP. Incidence of chronic laryngitis. Ann Otol Rhinol Laryngol. 2013;122:771–774. doi: 10.1177/000348941312201207. [DOI] [PubMed] [Google Scholar]
- 4.Langevin S, Michaud D, Marsit C, Nelson H, Birnbaum A, Eliot M, Christensen B, McClean M, Kelsey K. Gastric reflux is an independent risk factor for laryngopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2013;22:1061–1068. doi: 10.1158/1055-9965.EPI-13-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramachandran A, Snehalatha C. Rising burden of obesity in Asia. J Obes. 2010;2010:868573. doi: 10.1155/2010/868573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Serag H. The association between obesity and GERD: a review of the epidemiological evidence. Dig Dis Sci. 2008;53:2307–2312. doi: 10.1007/s10620-008-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farrokhi F, Vaezi MF. Laryngeal disorders in patients with gastroesophageal reflux disease. Minerva Gastroenterol Dietol. 2007;53:181–187. [PubMed] [Google Scholar]
- 8.Qua CS, Wong CH, Gopala K, Goh KL. Gastro-oesophageal reflux disease in chronic laryngitis: prevalence and response to acid-suppressive therapy. Aliment Pharmacol Ther. 2007;25:287–295. doi: 10.1111/j.1365-2036.2006.03185.x. [DOI] [PubMed] [Google Scholar]
- 9.Kweon S, Kim Y, Jang MJ, Kim Y, Kim K, Choi S, Chun C, Khang YH, Oh K. Data resource profile: The Korea National Health and Nutrition Examination Survey (KNHANES) Int J Epidemiol. 2014;43:69–77. doi: 10.1093/ije/dyt228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park HA. The Korea National Health and Nutrition Examination Survey as a primary data source. Korean J Fam Med. 2013;34:79. doi: 10.4082/kjfm.2013.34.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim Y, Park S, Kim NS, Lee BK. Inappropriate survey design analysis of the Korean National Health and Nutrition Examination Survey may produce biased results. J Prev Med Public Health. 2013;46:96–104. doi: 10.3961/jpmph.2013.46.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park SH, Lee KS, Park HY. Dietary carbohydrate intake is associated with cardiovascular disease risk in Korean: analysis of the third Korea National Health and Nutrition Examination Survey (KNHANES III) Int J Cardiol. 2010;139:234–240. doi: 10.1016/j.ijcard.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Coyle SM, Weinrich BD, Stemple JC. Shifts in relative prevalence of laryngeal pathology in a treatment-seeking population. J Voice. 2001;15:424–440. doi: 10.1016/S0892-1997(01)00043-1. [DOI] [PubMed] [Google Scholar]
- 14.Dworkin JP. Laryngitis: Types, causes, and treatments. Otolaryngol Clin North Am. 2008;41:419–436. doi: 10.1016/j.otc.2007.11.011. ix. [DOI] [PubMed] [Google Scholar]
- 15.Baletic N, Jakovljevic B, Marmut Z, Petrovic Z, Paunovic K. Chronic laryngitis in glassblowers. Ind Health. 2005;43:302–307. doi: 10.2486/indhealth.43.302. [DOI] [PubMed] [Google Scholar]
- 16.Chen H, Thornley P. Laryngeal tuberculosis: a case of a non-healing laryngeal lesion. Australas Med J. 2012;5:175–177. doi: 10.4066/AMJ.2012.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen CT, Merati AL. Acute and chronic laryngitis. In: Flint PW, Haughey BH, Lund VJ, Niparko JK, Robbins KT, Thomas JR, Lesperance MM, editors. Cummings Otolaryngology Head & Neck Surgery. Philadelphia: Mosby Elsevier; 2015. pp. 928–935. [Google Scholar]
- 18.Koufman JA. Laryngopharyngeal reflux is different from classic gastroesophageal reflux disease. Ear Nose Throat J. 2002;81:7–9. [PubMed] [Google Scholar]
- 19.Koufman JA, Aviv JE, Casiano RR, Shaw GY. Laryngopharyngeal reflux: position statement of the Committee on Speech, Voice, and Swallowing Disorders of the American Academy of Otolaryngology-Head and Neck Surgery. Otolaryngol Head Neck Surg. 2002;127:32–35. doi: 10.1067/mhn.2002.125760. [DOI] [PubMed] [Google Scholar]
- 20.Koufman JA. The otolaryngologic manifestations of gastroesophageal reflux disease (GERD): a clinical investigation of 225 patients using ambulatory 24-hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injury. Laryngoscope. 1991;101:1–78. doi: 10.1002/lary.1991.101.s53.1. [DOI] [PubMed] [Google Scholar]
- 21.Bitar K, Greenwood-Van Meerveld B, Saad R, Wiley JW. Aging and gastrointestinal neuromuscular function: insights from within and outside the gut. Neurogastroenterol Motil. 2011;23:490–501. doi: 10.1111/j.1365-2982.2011.01678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Besanko LK, Burgstad CM, Mountifield R, Andrews JM, Heddle R, Checklin H, Fraser RJ. Lower esophageal sphincter relaxation is impaired in older patients with dysphagia. World J Gastroenterol. 2011;17:1326–1331. doi: 10.3748/wjg.v17.i10.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nilsson M, Lundegardh G, Carling L, Ye W, Lagergren J. Body mass and reflux oesophagitis: an oestrogen-dependent association? Scand J Gastroenterol. 2002;37:626–630. doi: 10.1080/00365520212502. [DOI] [PubMed] [Google Scholar]
- 24.Nilsson M, Johnsen R, Ye W, Hveem K, Lagergren J. Obesity and estrogen as risk factors for gastroesophageal reflux symptoms. JAMA. 2003;290:66–72. doi: 10.1001/jama.290.1.66. [DOI] [PubMed] [Google Scholar]
- 25.Nordenstedt H, Zheng Z, Cameron AJ, Ye W, Pedersen NL, Lagergren J. Postmenopausal hormone therapy as a risk factor for gastroesophageal reflux symptoms among female twins. Gastroenterology. 2008;134:921–928. doi: 10.1053/j.gastro.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lagergren J. Influence of obesity on the risk of esophageal disorders. Nat Rev Gastroenterol Hepatol. 2011;8:340–347. doi: 10.1038/nrgastro.2011.73. [DOI] [PubMed] [Google Scholar]
- 27.Anggiansah R, Sweis R, Anggiansah A, Wong T, Cooper D, Fox M. The effects of obesity on oesophageal function, acid exposure and the symptoms of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2013;37:555–563. doi: 10.1111/apt.12208. [DOI] [PubMed] [Google Scholar]
- 28.Mercer CD, Rue C, Hanelin L, Hill LD. Effect of obesity on esophageal transit. Am J Surg. 1985;149:177–181. doi: 10.1016/s0002-9610(85)80029-5. [DOI] [PubMed] [Google Scholar]
- 29.Lee YC, Yen AM, Tai JJ, Chang SH, Lin JT, Chiu HM, Wang HP, Wu MS, Chen TH. The effect of metabolic risk factors on the natural course of gastro-oesophageal reflux disease. Gut. 2009;58:174–181. doi: 10.1136/gut.2008.162305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung SJ, Kim D, Park MJ, Kim YS, Kim JS, Jung HC, Song IS. Metabolic syndrome and visceral obesity as risk factors for reflux oesophagitis: a cross-sectional case-control study of 7078 Koreans undergoing health check-ups. Gut. 2008;57:1360–1365. doi: 10.1136/gut.2007.147090. [DOI] [PubMed] [Google Scholar]
- 31.Cholongitas E, Pipili C, Dasenaki M. Gastro-oesophageal reflux disease and irritable bowel syndrome significantly associated with metabolic syndrome. Scand J Gastroenterol. 2008;43:1405–1406. doi: 10.1080/00365520802308029. [DOI] [PubMed] [Google Scholar]
- 32.Dua K, Bardan E, Ren J, Sui Z, Shaker R. Effect of chronic and acute cigarette smoking on the pharyngoglottal closure reflex. Gut. 2002;51:771–775. doi: 10.1136/gut.51.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dua K, Bardan E, Ren J, Sui Z, Shaker R. Effect of chronic and acute cigarette smoking on the pharyngo-upper oesophageal sphincter contractile reflex and reflexive pharyngeal swallow. Gut. 1998;43:537–541. doi: 10.1136/gut.43.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moki F, Kusano M, Mizuide M, Shimoyama Y, Kawamura O, Takagi H, Imai T, Mori M. Association between reflux oesophagitis and features of the metabolic syndrome in Japan. Aliment Pharmacol Ther. 2007;26:1069–1075. doi: 10.1111/j.1365-2036.2007.03454.x. [DOI] [PubMed] [Google Scholar]