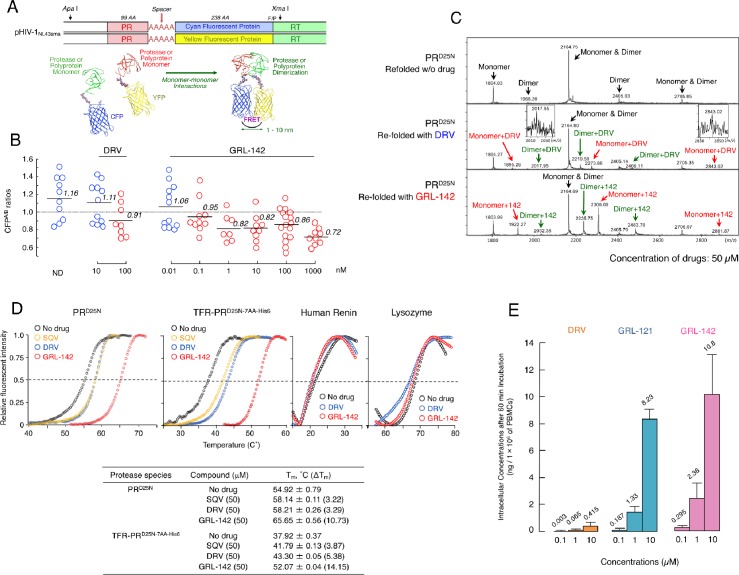
Figure 2. GRL-142 has significantly more potent HIV-1 protease dimerization inhibition activity, much greater thermal stability, and much higher intracellular concentration than DRV.
(A) The FRET-based HIV-1 expression assay system detecting HIV-1 PR dimerization and its disruption by DRV or GRL-142. In an attempt to elucidate the dynamics of HIV-1 PR dimerization and the mechanisms of the emergence of HIV-1 resistance against certain PR inhibitors (PIs), we developed an intermolecular FRET-based HIV-1 expression assay system using CYP- and YFP-tagged PR monomers. Using this FRET-based HIV-1 expression assay system, we have previously identified nonpeptidyl small-molecule inhibitors of HIV-1 PR dimerization, including DRV(Koh et al., 2007; Aoki et al., 2012). Various plasmids encoding full-length molecular infectious HIV-1 (HIV-1NL4-3) clones producing CFP- or YFP-tagged PR using the PCR-mediated recombination method were prepared. A linker consisting of five alanines was inserted between PR and fluorescent protein. A phenylalanine-proline site (F/P) that HIV-1 PR cleaves was also introduced between the fluorescent protein and reverse transcriptase. (Lower) Structural representations of PR monomers and dimer in association with the linker atoms and fluorescent proteins. FRET occurs only when the fluorescent proteins are 1–10 nm apart. (B) GRL-142 more potently inhibits PRWT dimerization by a factor of ~1000 than DRV. COS7 cells were exposed to various concentrations (0.01 to 1000 nM) of GRL-142 or DRV and were subsequently co-transfected with two plasmids, pHIV-PRWT-CFP and pHIV-PRWT-YFP, respectively. After 72 hr, cultured cells were examined in the FRET-based HIV-1 expression assay and the CFPA/B ratios (Y axis) were determined. The arithmetic mean values of the ratios obtained are shown as horizontal bars. A CFPA/B ratio that is greater than one signifies that protease dimerization occurred, whereas a ratio that is less than one signifies that the disruption of protease dimerization occurred (Koh et al., 2007; Aoki et al., 2012). All the experiments were conducted in a blind fashion. The P values were determined using the Wilcoxon rank-sum test (JMP software, SAS, Cary, NC) and were 0.6721 for the CFPA/B ratio in the absence of drug (CFPA/BNo-Drug) versus the CFPA/B ratio in the presence of 10 nM DRV (CFPA/B10-DRV), 0.0262 for CFPA/BNo-Drug versus CFPA/B100-DRV, 0.2483 for CFPA/BNo-Drug versus CFPA/B0.01- GRL-142, 0.0585 for CFPA/BNo-Drug versus CFPA/B0.1-GRL-142, 0.0145 for CFPA/BNo-Drug versus CFPA/B1-GRL-142, 0.0042 for CFPA/BNo-Drug versus CFPA/B10-GRL-142, 0.0056 for CFPA/BNo-Drug versus CFPA/B100-GRL-142, and 0.0019 for CFPA/BNo-Drug versus CFPA/B1000-GRL-142. (C) The ESI-MS spectrum of PRD25N in the absence or presence of 50 µM of DRV or GRL-142. Addition of DRV yielded two DRV-bound monomers and two DRV-bound dimers, while three GRL-142-bound monomers and three GRL-142-bound dimers were seen. Note that GRL-142 more tightly binds to monomers and dimers than DRV (by 6.78- and 3.13-fold; see the text), explaining at least in part the reason GRL-142 much more strongly blocks PR dimerization than DRV. (D) Thermal stability of PRD25N and TFR-PRD25N-7AA-His6 in the absence or presence of SQV, DRV, or GRL-142 was determined using the differential scanning fluorimetry. Tm (50% melting temperature) values were determined as the temperature at which the relative fluorescent intensity became 50%. Note that the thermal stability curves with GRL-142 significantly shifted to the higher temperature (to the right) than those with no agent, SQV, or DRV. The Tm values with GRL-142 were much higher than those with no agent, SQV, or DRV. The thermal stability curves of human renin and lysozyme did not shift at all with GRL-142 as compared with those with no agent, indicating highly specific binding of GRL-142 to PRD25N and TFR-PRD25N-7AA-His6. (E) GRL-142 achieves markedly higher intracellular concentration compared with DRV. PBMCs were incubated with 0.1, 1, and 10 μM of DRV, GRL-121, or GRL-142 for 60 min and vigorously washed with PBS and intra-cellular concentrations of each compound were determined using LC/MS. Arithmetic mean values shown are from the data derived from three independent experiments.