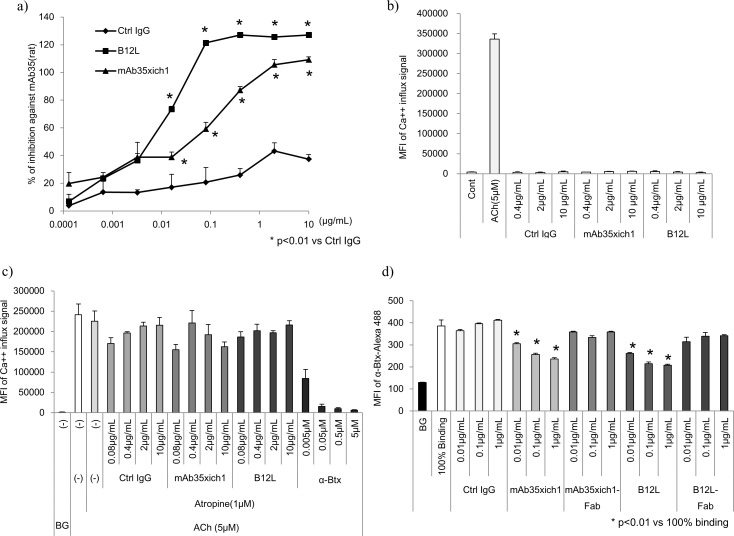
Fig 3. In vitro analysis of human B12L and rat mAb35.
a) Competitive binding of rat mAb35 was monitored by flow cytometer-based binding assay. The indicated concentrations of Abs (mAb35xich1, B12L and Ctrl IgG) were added to DB40 cells prior to spiking with 10 μg/mL of rat mAb35. Data was obtained by monitoring the mean fluorescence intensity (MFI) of anti-rat IgG-PE signal. The percent inhibition was calculated with the following formula: (MFI of rat mAb35 signal without blocking Abs–MFI of rat mAb35 signal with blocking Abs) / (MFI of rat mAb35 signal without blocking Abs–background signal) x 100 (%). All data were obtained from experiments performed in triplicate and are presented as mean values with SD. Ctrl IgG: isotype-matched human IgG for negative ctrl IgG. b) Agonistic and c) antagonistic activities of B12L/mAb35 were monitored by Ca++ influx in DB40 cells. Acetylcholine (ACh) was used as a positive control agonist. Atropine was used as an inhibitor of muscarinic type AChR to reduce the background signal for antagonist activity. α-Btx was used as a positive control antagonist. d) Downmodulation of nAChRs induced by B12L, mAb35xich1, and their Fabs was monitored by fluorescence signal of the α-Btx-Alexa Fluor 488 probe. Binding at 100% represents the maximum fluorescence signal of α-Btx-Alexa Fluor 488 bound to nAChRs on the surface of DB40 cells. BG: background signal. Statistical analysis among groups in a) and d) were conducted using Student’s t-test, with p < 0.01 being considered statistically significant.