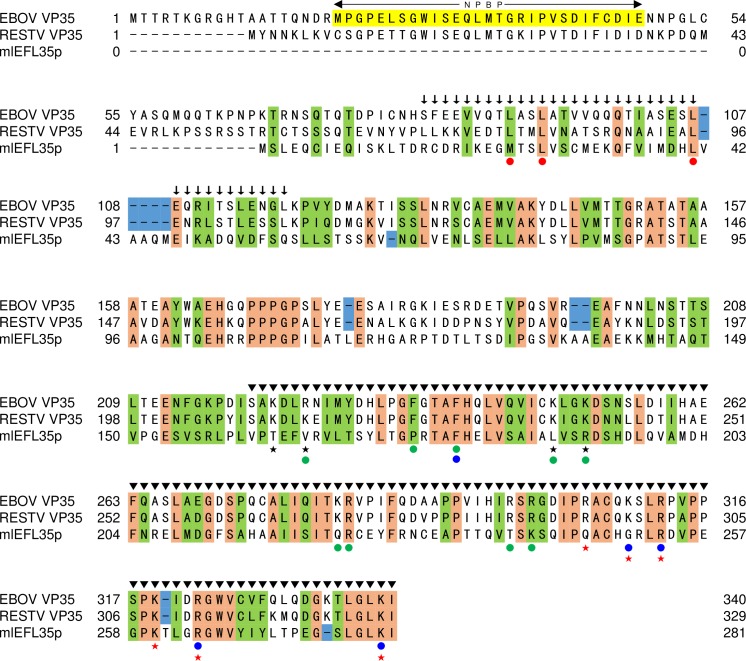
Fig 1. Comparison of primary structures of mlEFL35p and ebolavirus VP35s.
The same amino acid residues that are found in VP35s and mlEFL35p are highlighted in orange. Residues highlighted in green represent amino acids that are grouped together in the same classes, based on their physical/chemical properties. The blue rectangles show sequence gaps found between mlEFL35 and VP35s. The NP binding domain consisting of the residues 20–48 (EBOV numbering), termed NPBP, is highlighted in yellow. Amino acid residues indicated by red dots have been identified to be important for VP35 homo-oligomerization as well as viral replication and transcription [35]. Amino acid residues indicated by blue dots and green dots have been shown to be critical for the dsRNA binding and polymerase cofactor activities, respectively [31, 36]. The VP35 homo-oligomerization domain and IFN inhibitory domain are indicated by arrows and arrowheads, respectively. Asterisks indicate cysteine residues in VP35s. Black and red stars indicate the amino acids that form the FBP and the CBP regions on 3-dimensional structure of the VP35 IID, respectively.