Abstract
Infrageneric relations of the genetically diverse milkcap genus Lactifluus (Russulales, Basidiomycota) are poorly known. Currently used classification systems still largely reflect the traditional, mainly morphological, characters used for infrageneric delimitations of milkcaps. Increased sampling, combined with small-scale molecular studies, show that this genus is underexplored and in need of revision. For this study, we assembled an extensive dataset of the genus Lactifluus, comprising 80 % of all known species and 30 % of the type collections. To unravel the infrageneric relationships within this genus, we combined a multi-gene molecular phylogeny, based on nuclear ITS, LSU, RPB2 and RPB1, with a morphological study, focussing on five important characteristics (fruit body type, presence of a secondary velum, colour reaction of the latex/context, pileipellis type and presence of true cystidia). Lactifluus comprises four supported subgenera, each containing several supported clades. With extensive sampling, ten new clades and at least 17 new species were discovered, which highlight the high diversity in this genus. The traditional infrageneric classification is only partly maintained and nomenclatural changes are proposed. Our morphological study shows that the five featured characteristics are important at different evolutionary levels, but further characteristics need to be studied to find morphological support for each clade. This study paves the way for a more detailed investigation of biogeographical history and character evolution within Lactifluus.
Keywords: milkcaps, molecular evolution, morphology, taxonomy
INTRODUCTION
Russulales
Over the last two decades, molecular research strongly influenced and innovated our traditional view of the order Russulales (Larsson & Larsson 2003, Miller et al. 2006, Buyck et al. 2008). It soon became obvious that Friesian and other traditional classification systems overemphasised the phylogenetic importance of basidiocarp shape and hymenophore type. The genera Russula and Lactarius are different from other agaricoid mushrooms and hence were classified in their own order Russulales (Kreisel 1969, Oberwinkler 1977), among others supported by microscopic features such as sphaerocytes in the trama, amyloid spore ornamentation and a gloeoplerous hyphal system. As predicted, taxa with other basidiocarp types had to be included in this order (Romagnesi 1948, Donk 1971, Oberwinkler 1977, Larsson & Larsson 2003). Molecular data reveal strong support for a russuloid clade with corticioid, resupinate, discoid, effused-reflexed, clavarioid, pileate and sequestrate taxa with smooth, poroid, hydnoid, lamellate or labyrinthoid hymenophores, not all of them sharing sphaerocytes and amyloid spore ornamentation. There is morphological support for this Russulales clade in the presence of gloeocystidia or a gloeoplerous hyphal system (Larsson & Larsson 2003, Miller et al. 2006). Russula, Lactarius and some pleurotoid and sequestrate genera form an important group within this clade and are considered the Russulaceae Lotsy (Redhead & Norvell 1993, Miller et al. 2001, Larsson & Larsson 2003, Nuytinck et al. 2003, Eberhardt & Verbeken 2004).
Russulaceae
Generic concepts in the mushroom-forming Russulaceae changed when it became clear that pleurotoid, sequestrate and veiled forms originated several times, both in Lactarius and Russula. Morphological and molecular studies of pleurotoid Russulaceae species (Verbeken 1998b, Buyck & Horak 1999, Henkel et al. 2000), indicated that those species were placed within either Russula or Lactarius. Hence, the genus Pleurogala, which was erected to accommodate pleurotoid species formerly included in Lactarius sect. Panuoidei (Redhead & Norvell 1993), was abandoned. Sequestrate species also occur both in Lactarius (formerly placed in Arcangeliella, Gastrolactarius and Zelleromyces) and Russula (formerly placed in Cystangium, Elasmomyces, Gymnomyces, Martellia and Macowanites) (Calonge & Martín 2000, Miller et al. 2001, Binder & Bresinsky 2002, Desjardin 2003, Nuytinck et al. 2003, Eberhardt & Verbeken 2004, Lebel & Tonkin 2007, Verbeken et al. 2014). Species with a secondary velum occur both in Lactarius and Russula and were placed in separate genera (Hennings 1902, Heim 1937, Redhead & Norvell 1993), which was not accepted by Verbeken (1998b). Later, molecular analyses indicated that the Russulaceae family also contains several corticioid taxa from three genera: Boidinia, Gloeopeniophorella and Pseudoxenasma (Larsson & Larsson 2003, Miller et al. 2006). Lactarius and Russula species are ectomycorrhizal, the corticioid taxa are reported to be saprotrophic (Larsson & Larsson 2003, Miller et al. 2006, Tedersoo et al. 2010). However, this is questioned by Miller et al. (2006), who suggest that these corticioid taxa might also be ectomycorrhizal symbionts.
With the inclusion of more tropical taxa, phylogenetic data showed that Lactarius and Russula are not two well-defined and separate clades. Russula appears to be monophyletic only if a small group of species is excluded. This small group forms a clade where Lactarius and Russula are mixed and it was described as the new genus Multifurca (Buyck et al. 2008). The former Russula subsect. Ochricompactae, the Asian Russula zonaria and the American Lactarius furcatus were included in this genus. Multifurca species are characterised by furcate lamellae, dark yellowish lamellae and spore-prints, a strong zonation of pileus and context and the absence or presence of latex. The remainder of Lactarius falls in two different clades (Buyck et al. 2008). The proposal to conserve Lactarius (hereafter abbreviated as L.) with a conserved type L. torminosus (Buyck et al. 2010) was accepted by the 2011 International Botanical Congress (McNeill et al. 2011). The name Lactarius is therefore retained for the larger, mainly temperate clade. The subgenera L. subg. Lactarius (the former L. subg. Piperites), L. subg. Russularia and L. subg. Plinthogalus now constitute the larger genus Lactarius sensu novo. The smaller, mainly tropical clade, with approximately 150 described species (25 % of the known milkcap species), belongs to the genus Lactifluus (hereafter abbreviated as Lf.) and is typified by Agaricus lactifluus, currently known as Lf. volemus (Buyck et al. 2010). New combinations were made in a series of three papers for the subgenera Lf. subg. Lactariopsis, Lf. subg. Russulopsis, Lf. subg. Edules, Lf. subg. Gerardii, Lf. subg. Lactifluus and Lf. subg. Piperati (Verbeken et al. 2011, 2012, Stubbe et al. 2012b). No synapomorphic characteristics have been found to consistently separate the genera Lactarius and Lactifluus and the morphological distinction between the genera is thus far based on several trends. The genus Lactifluus is generally characterised by the complete absence of zonate and viscose to glutinose caps. It contains many species with veiled and velvety caps, as well as all known pleurotoid milkcap species (Buyck et al. 2008, Verbeken & Nuytinck 2013). So far, no sequestrate species are known within the genus Lactifluus.
Lactifluus
The milkcap genus Lactifluus is predominantly represented in the tropics. The highest diversity of the genus is known from Africa (Verbeken & Walleyn 2010) and Asia (Le et al. 2007b, Stubbe et al. 2010, Van de Putte et al. 2010), but recent studies indicate that the genus is also well-represented in South America (Henkel et al. 2000, Miller et al. 2002, Smith et al. 2011, Sá et al. 2013, Sá & Wartchow 2013). Typical host plants are leguminous trees (Fabaceae), members of the Dipterocarpaceae and the Fagaceae, and of the genera Uapaca (Phyllanthaceae), Eucalyptus and Leptospermum (Myrtaceae). Due to its mainly tropical distribution, the genus is rather understudied, but more and more species are recognised and described (Wang & Verbeken 2006, Van de Putte et al. 2010, 2012, De Crop et al. 2012, Miller et al. 2012, Stubbe et al. 2012a, Wang et al. 2012, Morozova et al. 2013, Sá et al. 2013, Sá & Wartchow 2013, Maba et al. 2014).
Lactifluus is known for its molecular diversity, with several species complexes (Stubbe et al. 2010, 2012a, Van de Putte et al. 2010, 2012, 2016, De Crop et al. 2014) and species on long and isolated branches (Buyck et al. 2007, Van de Putte et al. 2009, Morozova et al. 2013, Wang et al. 2015). Previous studies questioned the traditional subgenera and sections (Buyck et al. 2008) or even indicated that Lactifluus might be paraphyletic (Verbeken et al. 2014). These confusing results emphasize the need for a thorough study, since a genus-wide analysis of Lactifluus has never been published.
Current classification of Lactifluus
During the last decade, important changes were published regarding the infrageneric classification of the genus Lactifluus. The genus presently contains six subgenera and one unclassified section. A summarizing overview of the situation prior to our global phylogenetic analysis is given here.
Lactifluus subg. Lactariopsis
Lactifluus subg. Lactariopsis was traditionally divided into three sections: Lf. sect. Lactariopsis, Lf. sect. Chamaeleontini and Lf. sect. Albati (Verbeken 1998b, Verbeken et al. 2011). These sections were placed together especially based on similarities in pileipellis structure, such as the lack of a pseudoparenchymatous layer in combination with the presence of thick-walled hairs. In the phylogeny of Buyck et al. (2008), Lf. subg. Lactariopsis appears to be paraphyletic, with the temperate Lf. sect. Albati splitting off from the remaining, predominantly African part of the subgenus. Even though this was noticed, Lf. sect. Albati is still considered a section within Lf. subg. Lactariopsis by Verbeken et al. (2011) pending a more complete phylogenetic analysis. Lactifluus sect. Lactariopsis and Lf. sect. Chamaeleontini were originally separated based on the presence or absence of a secondary velum and the pileipellis structure (Verbeken 2001, Verbeken et al. 2012). However, the presence of a secondary velum seems to be of limited taxonomic value at this level, as molecular data show that species of both sections intermix in the phylogeny and the monophyly of neither section is supported (Buyck et al. 2007, 2008, Wang et al. 2015).
– Lactifluus sect. Albati occurs in temperate regions and consists of six known species with firm and white basidiocarps, a velutinous cap and acrid milk. Microscopically they can be recognised by a (lampro) trichoderm as pileipellis, pseudocystidia that are not emergent and the presence of macrocystidia (Heilmann-Clausen et al. 1998, Verbeken 1998b).
– Lactifluus sect. Chamaeleontini and Lf. sect. Lactariopsis mainly occur in tropical Africa, with some exceptions in South-East Asia and South America (Singer 1952, Verbeken & Horak 1999, Miller et al. 2012, Morozova et al. 2013). Species of Lf. sect. Chamaeleontini can be recognised by a pileipellis with scattered or absent thick-walled elements, the absence of secondary velum and emergent to highly emergent pseudocystidia. Species of Lf. sect. Lactariopsis are characterised by a pileipellis entirely composed of thick-walled elements, emergent to highly emergent pseudocystidia and the presence of a secondary velum, forming a clear annulus (Verbeken 1996a, 1998b, Verbeken & Walleyn 2010). Lactifluus sect. Lactariopsis also contains several pleurotoid species from South America and Southeast Asia (Verbeken 1998b, Miller et al. 2012, Morozova et al. 2013).
Lactifluus subg. Edules
This subgenus exclusively consists of African species, which are generally characterised by firm basidiocarps with yellowish to greyish orange to pinkish colours and a cap that is dry and often cracked, a trichoderm or trichopalisade as pileipellis and a spore ornamentation lower than 0.3 μm (Verbeken 1996a, Verbeken & Walleyn 1999, 2010). When it was described, the position of Lf. sect. Edules within the genus was uncertain (Verbeken 1995, 1996a) and later the section remained unclassified (Buyck et al. 2008). When recombining this section into Lactifluus, Verbeken et al. (2011) decided to treat this section on subgenus rank, as Lf. subg. Edules.
Lactifluus subg. Russulopsis
Verbeken (2001) and Verbeken et al. (2011) proposed this subgenus which includes only one section, Lf. sect. Russulopsidei, comprising eight species endemic to tropical Africa. Species are characterised by a dry to viscid pileus, reddish colours in pileus and stipe, and several striking microscopic features such as diverticulate and frequently branched pseudocystidia and a cutis-like pileipellis with distinct dermatocystidia, a character common in Russula but rarely observed in milkcaps (Verbeken 1996a, Verbeken & Walleyn 2010).
Lactifluus subg. Lactifluus
Lactifluus subg. Lactifluus is the largest subgenus and contains eight sections. The main characteristic of this subgenus is a palisade or palisade-like structure in the pileipellis.
– Lactifluus sect. Lactifluus contains species occurring throughout Europe, North America and Asia. Its members can be distinguished from species of other sections by a combination of several distinctive microscopic and macroscopic characteristics. Microscopically, they have a lampropalisade as pileipellis, hymenial lamprocystidia and reticulate spore ornamentation. Macroscopically, they can be recognised by clay-buff to orange-brown or reddish brown velutinous caps, abundant white latex that turns brownish when in contact with the flesh and a fish-like odour. Van de Putte et al. (2010, 2012, 2016) discovered a large diversity of cryptic to semi-cryptic species within this section.
– Lactifluus sect. Polysphaerophori is a predominantly African section, with only one South American representative, Lf. veraecrucis. Verbeken & Walleyn (2010) synonymised L. sect. Gymnocarpi with this section, as was also suggested by Montoya et al. (2007). The main characteristics are a strongly wrinkled pileus, a lampropalisade as pileipellis with a suprapellis thicker than the subpellis, the absence of true pleurocystidia, a more or less reticulate spore ornamentation, a hymenophoral trama mainly composed of sphaerocytes and a context that often changes green with FeSO4 (Verbeken 1996a, Verbeken & Walleyn 2010).
– Lactifluus sect. Phlebonemi is mainly represented by African species, although it contains some Asian and European representatives. It is characterised by spores with almost isolated rounded warts with some very fine connective lines and little to no reaction of the context with FeSO4 (Verbeken 1996a, Verbeken & Walleyn 2010). Similar to Lf. sect. Lactifluus they have latex that immediately changes brown and a fish-like odour, but they differ from that section by their hymenophoral trama mainly composed of narrow hyphae. The distinction between this section and Lf. sect. Polysphaerophori is mainly based on differences in spore ornamentation, but Verbeken & Walleyn (2010) state that this division might be artificial and was only conserved for practical reasons.
– Lactifluus sect. Pseudogymnocarpi contains seven species, which are all endemic to tropical Africa. The section is characterised by a lampropalisade as pileipellis, the presence of conspicuous lamprocystidia, elongate spores with a low incomplete to complete reticulum and often a central amyloid spot at the plage and a salmon pink reaction of the context with FeSO4 (Verbeken 1996a, Verbeken & Walleyn 2010).
– Lactifluus sect. Rubroviolascentini is a tropical African section containing two species characterised by a palisade as pileipellis, the presence of lamprocystidia, an extremely low spore ornamentation, an inamyloid plage and latex changing from white-buff, to red and finally black when exposed to air (Verbeken 1996a, Verbeken & Walleyn 2010). The section was distinguished from Lf. sect. Pseudogymnocarpi based on the blackening context. However, Verbeken & Walleyn (2010) note that this distinction is artificial and was only maintained for practical reasons.
– Lactifluus sect. Tomentosi contains species from Europe, Asia and Oceania, as Verbeken et al. (2012) synonymised L. sect. Rugati with this section. It can be recognised by a combination of characters: a dry and cracked pileus with yellow-orange to reddish brown colours, a palisade as pileipellis, a subpellis thicker than the suprapellis, the absence of true pleurocystidia, a more or less reticulate spore ornamentation, a hymenophoral trama mainly composed of sphaerocytes and a context that stains pink with FeSO4 (Verbeken 1996a, Verbeken & Walleyn 2010).
– Lactifluus sect. Tenuicystidiati is an Asian section, recently proposed by Wang et al. (2015). The type of this section was originally placed in L. sect. Pseudogymnocarpi, by Wang & Verbeken (2006) due to the morphological similarity to some species of that section. However, this was not supported by molecular results, which suggested a closer affinity with Lf. sect. Lactifluus. Because of the clear morphological delimitation between Lf. sect. Tenuicystidiati and Lf. sect. Lactifluus, this group is now treated as a new section, sister to Lf. sect. Lactifluus (Wang et al. 2015). It is characterised by a combination of characteristics: a lampropalisade as pileipellis with slightly thick-walled terminal cells, thin-walled and slender macrocystidia and ellipsoid spores with low and more or less connected ornamentation.
– Lactifluus sect. Ambicystidiati currently contains only one species known from Asia, Lf. ambicystidiatus. This species shows a combination of striking characteristics: an undeveloped lactiferous system and the presence of both macro- and lamprocystidia. Wang et al. (2015) treated Lf. sect. Ambicystidiati as an independent section within the genus Lactifluus, as this species shows no morphological similarity with any other taxon within the subgenus.
Lactifluus subg. Gerardii
Due to striking morphological similarities, Lf. gerardii and allies were long considered to belong to L. subg. Plinthogalus (Hesler & Smith 1979). Using a combination of molecular and morphological data, Stubbe et al. (2010) found that they form a separate group and actually belong to the genus Lactifluus instead of Lactarius. These species were transferred to Lf. subg. Gerardii, which contains up to 30 described species. The subgenus is distributed in Asia, North and Central America and Australasia. In most cases species in Lf. subg. Gerardii can be recognised by a combination of five characteristics: a white spore print, reticulate spore ornamentation not higher than 2 μm, a palisade structure in the pileipellis with globose cells in the subpellis, the lack of macrocystidia and a general habitus of a brown pileus and stipe with contrasting white and mostly distant lamellae (Stubbe et al. 2010). This subgenus also contains several pleurotoid species that are morphologically different, because they lack the general habitus and the striking dark pigmentation of this subgenus and have macrocystidia in their hymenium.
Lactifluus subg. Piperati
This subgenus with a Northern hemispherical distribution contains two sections: Lf. sect. Piperati and Lf. sect. Allardii. Lactifluus sect. Piperati contains at least 10 different species distributed over three groups (De Crop et al. 2014) and all of them are characterised by firm, whitish basidiocarps and a hyphoepithelium as pileipellis type with dermatocystidia (Heilmann-Clausen et al. 1998). Lactifluus sect. Allardii contains only one North American species and can be recognised by a lamprotrichoderm as pileipellis and a vinaceous-cinnamon coloured pileus (Hesler & Smith 1979).
Unclassified section
Lactifluus sect. Aurantiifolii has not been placed in a subgenus. The section contains only one African representative, Lf. aurantiifolius, that deviates morphologically from all other milkcap species and is characterised by a slightly velutinous to pruinose, vividly coloured and concentrically zonate pileus, brightly coloured lamellae with a paler and fimbriate margin, irregularly verrucose to incompletely reticulate spores, clavate pleuromacrocystidia with slightly thickened walls and a trichoderm pileipellis structure (Verbeken 1996b, Buyck et al. 2007). In previous studies, the classification of this section was uncertain (Buyck et al. 2007, Verbeken et al. 2012).
Unclassified species
Some Lactifluus species have unclear taxonomic positions, such as the agaricoid Lf. caperatus and Lf. cocosmus from Africa and the Australian Lf. subclarkeae; and the pleurotoid Neotropical Lf. multiceps, Lf. brunellus and Lf. panuoides.
This study is the first worldwide treatment of the genus Lactifluus, with a thorough geographical and taxonomical sampling. We combine a multi-gene molecular phylogeny with a morphological approach to clarify relationships within Lactifluus. The current classification is compared with our results, nomenclatural changes are listed and we give an overview of the revised infrageneric classification.
MATERIAL AND METHODS
Sampling
We included Lactifluus collections from every continent, every subgenus and every section, as well as collections with divergent morphological features. To improve species identification, we included as many type specimens as possible in our dataset. We included one collection of each species, except when sequences of only one or two genes of the type collection were available. In those cases we added an extra collection of the same species for which all four genes were sequenced. The outgroup contains nine Russulales species: Amylostereum laevigatum, Auriscalpium vulgare, Bondarzewia montana, Echinodontium tinctorium, Gloeocystidiellum porosum, Heterobasidion annosum, Peniophora nuda, Stereum hirsutum and Vararia abortiphysa (Table 1).
Table 1.
Specimens and GenBank accession numbers of DNA sequences used in the molecular analyses. The arrangement of the subgenera and sections in the table follows their position in the concatenated phylogeny of the genus Lactifluus (Fig. 1).
| Species | Voucher collection (herbarium) | Country | GenBank Accession numbers |
|||
|---|---|---|---|---|---|---|
| ITS | LSU | RPB2 | RPB1 | |||
| Genus Lactifluus | ||||||
| Lactifluus subg. Lactariopsis | ||||||
| Lactifluus sect. Lactariopsis | ||||||
| Lactifluus annulatoangustifolius | BB 00-1518 (GENT, PC) | Madagascar | AY606981 | KR364253 | – | – |
| Lactifluus cf. zenkeri | AV 11-050 (GENT) | Tanzania | KR364055 | KR364182 | KR364297 | KR364425 |
| Lactifluus chamaeleontinus | JD 946 (BR) | Congo | KR364079 | KR364208 | KR364267 | KR364377 |
| Lactifluus heimii | EDC 11-082 (GENT) | Tanzania | KR364040 | KR364167 | KR364286 | KR364412 |
| Lactifluus heimii Type | AV 94-465 (GENT) | Burundi | KR364025 | KR364152 | – | – |
| Lactifluus laevigatus | JD 939 (BR) | Congo | KR364077 | KR364206 | KR364290 | KR364417 |
| Lactifluus pelliculatus | JD 956 (BR) | Congo | KR364080 | KR364209 | KR364321 | KR364449 |
| Lactifluus pruinatus Type | BB 3248 (GENT) | Zambia | KR364031 | KR364158 | KR364328 | KR364458 |
| Lactifluus sesemotani | AV 94-476 (GENT) | Burundi | KR364036 | KR364163 | KR364345 | KR364476 |
| Lactifluus sp. | EDC 12-040 (GENT) | Cameroon | KR364063 | KR364192 | KR364289 | KR364416 |
| Lactifluus uapacae Type | AV 07-048 (GENT) | Cameroon | KR364007 | KR364135 | KR364352 | KR364483 |
| Lactifluus velutissimus | JD 886 (BR) | Congo | KR364075 | KR364204 | KR364355 | KR364485 |
| Clade 1 | ||||||
| Lactifluus emergens | AV 99-012 (GENT) | Zimbabwe | KR364021 | KR364148 | KR364276 | KR364388 |
| Lactifluus madagascariensis | BB 99-409 (PC) | Madagascar | AY606977 | DQ421975 | DQ421914 | – |
| Lactifluus madagascariensis Type | B-E 99-417 (GENT) | Madagascar | KR364120 | KR364245 | – | – |
| Isolated species 1 | ||||||
| Lactifluus acrissimus | EDC 11-112 (GENT) | Tanzania | KR364041 | KR364168 | KR364254 | KR364366 |
| Lactifluus acrissimus Type | ADK2161 (GENT) | Benin | KR364126 | – | – | – |
| Clade 2 | ||||||
| Lactifluus annulifer | TH 9014 (BRG, DUKE) | Guyana | KC155376 | KC155376 | – | – |
| Lactifluus sp. | RC/Guy 09-004bis (LIP) | French Guiana | KJ786643 | KP691419 | KP691427 | – |
| Lactifluus subiculatus | SLM 10114 (BRG, RMS) | Guyana | JQ405654 | – | – | – |
| Lactifluus venezuelanus | RC/Guad 11-017 (LIP) | Guadeloupe | KP691411 | KP691420 | KP691429 | KR364393 |
| Clade 3 | ||||||
| Lactifluus multiceps | TH 9154A (BRG, DUKE) | Guyana | JN168731 | – | – | – |
| Lactifluus sp. | G3264 (MNHN) | French Guiana | KJ786706 | KJ786620 | KP691435 | KR364400 |
| Clade 4 | ||||||
| Lactifluus chrysocarpus Type | LE 253907 (LE) | Vietnam | JX442761 | JX442761 | – | – |
| Lactifluus ramipilosus Type | EDC 14-503 (GENT, MFLU) | Thailand | KR364128 | – | – | – |
| Clade 5 | ||||||
| Lactifluus brachystegiae Type | AV 99-002 (GENT) | Zimbabwe | KR364018 | KR364145 | KR364262 | KR364374 |
| Lactifluus leoninus | DS 07-454 (GENT) | Thailand | KF220055 | JN388989 | JN375592 | JN389188 |
| Lactifluus leoninus Type | EH 72-524 (GENT) | Papua New Guinea | KR364116 | – | – | – |
| Lactifluus sp. | AV 11-183 (GENT) | Togo | KR364060 | KR364189 | KR364277 | KR364389 |
| Isolated species 2 | ||||||
| Lactifluus cocosmus Type | ADK 4462 (GENT) | Togo | KR364013 | KR364141 | KR364269 | KR364380 |
| Clade 6 | ||||||
| Lactifluus rufomarginatus | ADK 3358 (BR) | Benin | KR364033 | KR364160 | KR364335 | KR364466 |
| Lactifluus rufomarginatus Type | ADK 3011 (GENT) | Benin | KR364034 | KR364161 | KR364336 | – |
| Lactifluus sp. | AV 07-056 (GENT) | Cameroon | KR364008 | KR364136 | KR364293 | KR364421 |
| Lactifluus sp. | EDC 12-195 (GENT) | Cameroon | KR364071 | KR364200 | KR364301 | KR364429 |
| Clade 7 | ||||||
| Lactifluus densifolius | AV 11-111 (GENT) | Tanzania | KR364057 | KR364184 | KR364273 | KR364385 |
| Lactifluus sp. | JD 907 (GENT) | Congo | KR364076 | KR364205 | KR364302 | KR364430 |
| Lactifluussect. Russulopsidei | ||||||
| Lactifluus cyanovirescens | JD 988 (GENT) | Congo | KR364082 | KR364211 | KR364270 | KR364382 |
| Lactifluus longipes | JD 303 (BR) | Gabon | KR364009 | KR364137 | KR364310 | KR364438 |
| Lactifluus ruvubuensis | AB 305 (GENT) | Guinea | KR364035 | KR364162 | KR364343 | KR364473 |
| Lactifluus ruvubuensis Type | AV 94-599 (GENT) | Burundi | KR364122 | – | – | – |
| Lactifluus urens | EDC 14-032 (GENT) | Zambia | KR364124 | KR364247 | KR364353 | – |
| Lactifluus sect. Edules | ||||||
| Lactifluus aureifolius | AV 11-074 (GENT) | Tanzania | KR364056 | KR364183 | KR364259 | KR364371 |
| Lactifluus edulis | FN 05-628 (GENT) | Malawi | KR364020 | KR364147 | KR364275 | KR364387 |
| Lactifluus fazaoensis Type | AV 11-178 (GENT) | Togo | HG426477 | KR364188 | KR364349 | KR364481 |
| Lactifluus indusiatus Type | AV 94-122 (GENT) | Burundi | KR364026 | KR364153 | KR364287 | – |
| Lactifluus inversus | AB 063 (GENT) | Guinea | AY606976 | DQ421978 | DQ421917 | KR364414 |
| Lactifluus latifolius | SDM 037 (BR) | Gabon | KR364028 | KR364155 | KR364291 | KR364418 |
| Lactifluus nodosicystidiosus | BEM 97-273 (GENT) | Madagascar | KR364029 | KR364156 | KR364316 | KR364444 |
| Lactifluus nodosicystidiosus Type | BEM 97-072 (GENT) | Madagascar | AY606975 | DQ421976 | DQ421915 | – |
| Lactifluus phlebophyllus | BB 00-1388 (PC) | Madagascar | AY606974 | DQ421979 | DQ421918 | – |
| Lactifluus roseolus | AV 99-160 (GENT) | Zimbabwe | KR364032 | KR364159 | KR364333 | KR364463 |
| Lactifluus roseolus Type | AV 94-274 (GENT) | Burundi | KR364121 | KR364242 | – | – |
| Lactifluus sp. nov. | EDC 12-068 (GENT) | Cameroon | KR364068 | KR364197 | KR364299 | KR364427 |
| Lactifluus sect. Albati | ||||||
| Lactifluus bertillonii | JN 2012-016 (GENT) | Germany | KR364087 | KR364217 | KR364261 | KR364373 |
| Lactifluus deceptivus | TENN 065854 (TENN) | North America | KR364101 | – | KR364271 | KR364383 |
| Lactifluus pilosus Type | LTH 205 (GENT) | Thailand | KR364006 | KR364134 | KR364323 | KR364452 |
| Lactifluus sp. nov. | JN 2011-071 (GENT) | Vietnam | KR364043 | KR364169 | KR364255 | KR364367 |
| Lactifluus sp. nov. | JN 2011-077 (GENT) | Vietnam | KR364044 | KR364170 | KR364256 | KR364368 |
| Lactifluus subvellereus | AV 05-210 (GENT) | North America | KR364010 | KR364138 | KR364347 | KR364479 |
| Lactifluus vellereus | ATHU-M 8077 (ATHU-M) | Greece | KR364106 | KR364237 | KR364354 | KR364484 |
| Lactifluus subg. Pseudogymnocarpi | ||||||
| Lactifluus sect. Pseudogymnocarpi | ||||||
| Lactifluus cf. longisporus | AV 11-025 (GENT) | Tanzania | KR364054 | KR364181 | KR364311 | KR364439 |
| Lactifluus cf. pseudogymnocarpus | AV 05-085 (GENT) | Malawi | KR364012 | KR364139 | KR364329 | KR364459 |
| Lactifluus cf. pumilus | EDC 12-066 (GENT) | Cameroon | KR364067 | KR364196 | KR364332 | KR364462 |
| Lactifluus gymnocarpoides | JD 885 (BR) | Congo | KR364074 | KR364203 | KR364283 | KR364409 |
| Lactifluus gymnocarpoides | AV 05-184 (GENT) | Malawi | KR364024 | KR364151 | KR364284 | KR364410 |
| Lactifluus hygrophoroides | AV 05-251 (GENT) | North America | HQ318285 | HQ318208 | HQ328936 | KR364413 |
| Lactifluus longisporus Type | AV 94-557 (GENT) | Burundi | KR364118 | KR364244 | – | – |
| Lactifluus luteopus | EDC 11-087 (GENT) | Tanzania | KR364049 | KR364176 | KR364312 | KR364441 |
| Lactifluus luteopus Type | AV 94-463 (GENT) | Burundi | KR364119 | – | KR364313 | – |
| Lactifluus medusae | EDC 12-152 (GENT) | Cameroon | KR364069 | KR364198 | KR364314 | KR364442 |
| Lactifluus pseudoluteopus | FH 12-026 (GENT) | Thailand | KR364084 | KR364214 | KR364331 | KR364460 |
| Lactifluus rugatus | EP 1212/7 (LGAM-AUA) | Greece | KR364104 | KR364235 | KR364337 | KR364467 |
| Lactifluus sudanicus Type | AV 11-174 (GENT) | Togo | HG426469 | KR364186 | KR364348 | KR364480 |
| Lactifluus sect. Xerampelini | ||||||
| Lactifluus cf. pseudovolemus | ADK 2927 (GENT) | Benin | KR364113 | KR364243 | KR364330 | KR364461 |
| Lactifluus goossensiae | AB 320 (GENT) | Guinea | KR364132 | KR364252 | KR364281 | – |
| Lactifluus kivuensis Type | JR Z 310 (GENT) | Congo | KR364027 | KR364154 | – | – |
| Lactifluus rubiginosus | JD 959 (BR) | Congo | KR364081 | KR364210 | KR364304 | KR364432 |
| Lactifluus rubiginosus Type | BB 3466 (GENT) | Zambia | KR364014 | KR364250 | – | – |
| Lactifluus sp. nov. | EDC 12-001 (GENT) | Cameroon | KR364061 | KR364190 | KR364298 | KR364426 |
| Lactifluus sp. nov. | EDC 12-176 (GENT) | Cameroon | KR364070 | KR364199 | KR364300 | KR364428 |
| Lactifluus xerampelinus | MH 201176 (GENT) | Mozambique | KR364099 | KR364231 | KR364364 | KR364496 |
| Lactifluus xerampelinus Type | TS 1116 (GENT) | Tanzania | KR364039 | KR364166 | – | – |
| Clade 8 | ||||||
| Lactifluus armeniacus Type | EDC 14-501 (GENT, MFLU) | Thailand | KR364127 | – | – | – |
| Lactifluus sp. nov. | JN 2011-012 (GENT) | Vietnam | KR364045 | KR364171 | KR364294 | KR364422 |
| Lactifluus sp. nov. | TENN 065929 (TENN) | North America | KR364102 | KR364233 | KR364308 | KR364436 |
| Lactifluus volemoides | MH 201187 (GENT) | Mozambique | KR364098 | KR364230 | KR364363 | KR364493 |
| Lactifluus volemoides Type | TS 0705 (GENT) | Tanzania | KR364038 | KR364165 | – | – |
| Lactifluus sect. Aurantiifolii | ||||||
| Lactifluus aurantiifolius Type | AV 94-063 (GENT) | Burundi | KR364017 | KR364144 | – | – |
| Lactifluus sect. Rubroviolascentini | ||||||
| Lactifluus aff. rubroviolascens | EDC 12-051 (GENT) | Cameroon | KR364066 | KR364195 | KR364334 | KR364465 |
| Lactifluus carmineus Type | AV 99-099 (GENT) | Zimbabwe | KR364131 | KR364251 | KR364265 | – |
| Lactifluus denigricans | EDC 11-218 (GENT) | Tanzania | KR364051 | KR364178 | KR364272 | KR364384 |
| Lactifluus sp. nov. | AV 11-006 (GENT) | Tanzania | KR364052 | KR364179 | KR364288 | KR364415 |
| Lactifluus kigomaensis | EDC 11-159 (GENT) | Tanzania | KR364050 | KR364177 | KR364295 | KR364423 |
| Lactifluus sect. Polysphaerophori | ||||||
| Lactifluus pegleri | PAM/Mart 12-091 (LIP) | Martinique | KP691416 | KP691425 | KP691433 | KR364397 |
| Lactifluus sp. | RC/Guy 09-036 (LIP) | French Guiana | KJ786645 | KJ786550 | KP752178 | – |
| Lactifluus sp. | MR/Guy 13-145 | French Guiana | KJ786691 | KJ786595 | KP752180 | KR364398 |
| Lactifluus sp. | MCA 3937 (GENT) | Guyana | KR364109 | KR364240 | KR364350 | – |
| Lactifluus veraecrucis Type | M 8025 (ENCB) | Mexico | KR364112 | KR364241 | – | – |
| Lactifluus subg. Gymnocarpi | ||||||
| Lactifluus sect. Luteoli | ||||||
| Lactifluus brunneoviolascens | AV 13-038 (GENT) | Italy | KR364123 | KR364246 | KR364264 | KR364376 |
| Lactifluus longivelutinus Type | XHW 1565 (GENT) | China | KR364114 | – | – | – |
| Lactifluus luteolus | AV 05-253 (GENT) | North America | KR364016 | KR364142 | KJ210067 | KR364440 |
| Lactifluus nonpiscis | AV 11-137 (GENT) | Togo | KR364058 | KR364185 | KR364317 | KR364445 |
| Lactifluus nonpiscis Type | BB 3171 (GENT) | Zambia | KR364030 | KR364157 | – | – |
| Lactifluus rubrobrunnescens Type | EH 7194 (GENT) | Indonesia | KR364115 | – | – | – |
| Lactifluus sp. nov. | KW 392 (GENT) | Thailand | KR364091 | KR364222 | KR364305 | KR364433 |
| Lactifluus sp. nov. | REH 9398 (NY) | Australia | KR364097 | KR364229 | KR364307 | KR364435 |
| Lactifluus sect. Gymnocarpi | ||||||
| Lactifluus albocinctus Type | AV 99-211 (GENT) | Zimbabwe | KR364117 | KR364249 | KR364258 | – |
| Lactifluus albomembranaceus nom. prov. | EDC 12-046 (GENT) | Cameroon | KR364064 | KR364193 | KR364257 | KR364369 |
| Lactifluus flammans | JD 941 (BR) | Congo | KR364078 | KR364207 | KR364303 | KR364431 |
| Lactifluus gymnocarpus | EDC 12-047 (GENT) | Cameroon | KR364065 | KR364194 | KR364282 | KR364408 |
| Lactifluus cf. tanzanicus | AV 11-017 (GENT) | Tanzania | KR364053 | KR364180 | KR364296 | KR364424 |
| Lactifluus tanzanicus Type | TS 1277 (GENT) | Tanzania | KR364037 | KR364164 | KR364351 | – |
| Isolated species 4 | ||||||
| Lactifluus foetens | ADK 3688 (BR) | Benin | KR364022 | KR364149 | KR364278 | KR364390 |
| Lactifluus foetens Type | ADK 2840 (BR) | Benin | KR364023 | KR364150 | KR364279 | KR364391 |
| Lactifluus sect. Phlebonemi | ||||||
| Lactifluus aff. phlebonemus | EDC 12-023 (GENT) | Cameroon | KR364062 | KR364191 | KR364322 | KR364451 |
| Lactifluus brunnescens | AV 05-083 (GENT) | Malawi | KR364019 | KR364146 | KR364263 | KR364375 |
| Clade 9 | ||||||
| Lactifluus aff. nebulosus | RC/Guad 11-023 (LIP) | Guadeloupe | KP691412 | KP691421 | KP691430 | KR364394 |
| Lactifluus caribaeus | PAM/Mart 12-090 (LIP) | Martinique | KP691415 | KP691424 | KP691432 | KR364396 |
| Lactifluus cf. castaneibadius | CL/MART06.019 (LIP) | Martinique | KP691417 | KP691426 | – | – |
| Lactifluus cf. murinipes | F.1890 (LIP) | Martinique | KP691418 | – | – | – |
| Lactifluus cf. putidus | PAM/Mart 11-013 (LIP) | Martinique | KP691413 | KP691422 | KP691431 | KR364395 |
| Lactifluus chiapanensis | VMB 4374A (GENT) | Mexico | GU258297 | GU265580 | GU258316 | KR364378 |
| Isolated species 5 | ||||||
| Lactifluus sp. | G3185 | French Guiana | KJ786694 | KJ786603 | KP691434 | KR364399 |
| Isolated species 6 | ||||||
| Lactifluus brunellus | TH 9130 (BRG, DUKE) | Guyana | JN168728 | – | – | – |
| Isolated species 7 | ||||||
| Lactifluus sp. | RC/Guad 08-042 (LIP) | Guadeloupe | KP691414 | KP691423 | KP752179 | – |
| Isolated species 8 | ||||||
| Lactifluus panuoides | RC/Guy 10-024 (LIP) | French Guiana | KJ786647 | KJ786551 | KP691428 | – |
| Lactifluus sect. Tomentosi | ||||||
| Lactifluus clarkeae | MN 2004002 (L) | Australia | KR364011 | HQ318205 | KR364268 | KR364379 |
| Lactifluus flocktonae | JET1006 (MEL) | Australia | JX266621 | JX266637 | – | – |
| Lactifluus sp. | PGK13-130 | New Caledonia | KP691436 | KR605507 | – | – |
| Lactifluus subclarkeae | REH 9231 (NY) | Australia | KR364095 | KR364227 | KR364346 | KR364477 |
| Lactifluus subg. Lactifluus | ||||||
| Lactifluus sect. Lactifluus | ||||||
| Lactifluus acicularis | KVP 08-002 (GENT) | Thailand | HQ318226 | HQ318132 | HQ328869 | JN389131 |
| Lactifluus corrugis s.l. | AV 05-392 (GENT) | North America | JQ753822 | KR364143 | JQ348127 | – |
| Lactifluus crocatus | KVP 08-034 (GENT) | Thailand | HQ318243 | HQ318151 | HQ328888 | JN389145 |
| Lactifluus dissitus | AV-KD-KVP 09-134 (GENT) | India | JN388978 | JN389026 | JN375628 | JN389172 |
| Lactifluus distantifolius | LTH 288 (GENT) | Thailand | HQ318274 | HQ318193 | KR364274 | JN389155 |
| Lactifluus lamprocystidiatus Type | EH 72-195 (GENT) | Papua New Guinea | KR364015 | – | – | – |
| Lactifluus leptomerus Type | AV-KD-KVP 09-131 (GENT) | India | JN388972 | JN389023 | JN375625 | JN389169 |
| Lactifluus longipilus | LTH 184 (GENT) | Thailand | HQ318256 | HQ318169 | HQ328905 | JN389152 |
| Lactifluus oedematopus | KVP 12-001 (GENT) | Germany | KR364100 | KR364232 | KR364319 | KR364447 |
| Lactifluus pinguis Type | AV-RW 04-023/LTH117 (GENT) | Thailand | HQ318211 | HG318111 | HQ328858 | JN389126 |
| Lactifluus sp. | SA A12 L2 (GENT) | North America | KR364088 | KR364218 | KR364361 | KR364491 |
| Lactifluus subvolemus | KVP 08-048 (GENT) | Slovenia | JQ753927 | JQ348379 | KR364356 | KR364486 |
| Lactifluus versiformis Type | AV-KD-KVP 09-045 (GENT) | India | JN388967 | JN389031 | JN375632 | JN389177 |
| Lactifluus vitellinus | KVP 08-024 (GENT) | Thailand | HQ318236 | HQ318144 | HQ328881 | JN389138 |
| Lactifluus volemus | KVP 11-002 (GENT) | Belgium | JQ753948 | KR364175 | KR364360 | KR364490 |
| Lactifluus volemus s.l. | AV-KD-KVP 09-121 (GENT) | India | JN388979 | JN389014 | JN375616 | JN389160 |
| Lactifluus volemus s.l. | KVP 08-011 (GENT) | Thailand | HQ318232 | HQ318139 | HQ328876 | JN389135 |
| Lactifluus volemus s.l. | KVP 08-031 (GENT) | Thailand | HQ318240 | HQ318148 | HQ328885 | JN389142 |
| Lactifluus volemus s.l. | REH 9320 (NY) | Australia | KR364096 | KR364228 | KR364362 | KR364492 |
| Lactifluus sect. Tenuicystidiati | ||||||
| Lactifluus aff. tenuicystidiatus | JN 2011-074 (GENT) | Vietnam | KR364047 | KR364173 | KR364358 | KR364488 |
| Lactifluus sp. | JN 2011-080 (GENT) | Vietnam | KR364048 | KR364174 | KR364359 | KR364489 |
| Lactifluus subpruinosus | JN 2011-061 (GENT) | Vietnam | KR364046 | KR364172 | KR364357 | KR364487 |
| Lactifluus sect. Gerardii | ||||||
| Lactifluus atrovelutinus | DS 06-003 (GENT) | Malaysia | GU258231 | GU265588 | GU258325 | JN389185 |
| Lactifluus conchatulus Type | LTH 457 (GENT) | Thailand | GU258296 | GU265659 | GU258399 | KR364381 |
| Lactifluus fuscomarginatus Type | LM 4379 (XAL) | Mexico | HQ168367 | HQ168367 | – | – |
| Lactifluus genevievae Type | GG-DK 17-02-05 (GENT) | Australia | GU258294 | GU265657 | GU258397 | KR364401 |
| Lactifluus aff. gerardii | LTH 270 (GENT) | Thailand | EF560685 | GU265598 | GU258335 | KR364402 |
| Lactifluus gerardii | AV 05-375 (GENT) | North America | GU258254 | GU265616 | GU258353 | KR364403 |
| Lactifluus cf. gerardii var. fagicola | JN 2007-029 (GENT) | Canada | GU258224 | GU265582 | GU258318 | – |
| Lactifluus igniculus Type | LE 262983 (LE) | Vietnam | JX442759 | JX442759 | – | – |
| Lactifluus leae | FH 12-013 (GENT) | Thailand | KF432957 | KR364213 | KR364292 | KR364419 |
| Lactifluus leonardii | GG 07-02-04 | Australia | GU258308 | GU265668 | GU258408 | KR364495 |
| Lactifluus limbatus Epitype | DS 06-247 (GENT) | Malaysia | JN388955 | JN388987 | JN375590 | JN389186 |
| Lactifluus cf. ochrogalactus | AV-KD-KVP 09-120 (GENT) | India | KR364130 | KR364248 | KR364318 | KR364446 |
| Lactifluus petersenii | AV 05-300 (GENT) | North America | GU258281 | GU265642 | GU258382 | KR364450 |
| Lactifluus reticulatovenosus Type | EH 6472 (GENT) | Indonesia | GU258286 | GU265649 | GU258389 | – |
| Lactifluus sp. nov. | AV 12-050 (GENT) | Thailand | KR364086 | KR364216 | KR364260 | KR364372 |
| Lactifluus sp. nov. | AV 12-070 (GENT) | Thailand | KR364090 | KR364221 | KR364326 | – |
| Lactifluus sp. nov. | TENN 051830 (TENN) | Nepal | KR364111 | KR364140 | – | – |
| Lactifluus sp. nov. | KW 304/FH 12-037 (GENT) | Thailand | KR364092 | KR364223 | KR364306 | KR364434 |
| Lactifluus subgerardii | AV 05-269 (GENT) | North America | GU258263 | GU265625 | GU258362 | KR364478 |
| Lactifluus wirrabara s.l. | PL 40509 | New Zealand | GU258287 | GU265650 | GU258390 | KR364475 |
| Lactifluus wirrabara s.l. | GG 24-01-04 | Australia | GU258307 | GU265667 | GU258407 | KR364494 |
| Lactifluus sect. Ambicystidiati | ||||||
| Lactifluus ambicystidiatus | HKAS J7008 (HKAS) | China | KR364108 | KR364239 | KR364309 | KR364437 |
| Isolated species 9 | ||||||
| Lactifluus sp. nov. | PUN 7046 (PUN) | India | KM658971 | – | – | – |
| Lactifluus sect. Allardii | ||||||
| Lactifluus allardii | JN 2004-008 (GENT) | North America | KF220016 | KF220125 | KF220217 | KR364370 |
| Lactifluus sect. Piperati | ||||||
| Lactifluus aff. glaucescens | AV 04-195 (GENT) | North America | KF220045 | KF220146 | KF220232 | KR364404 |
| Lactifluus aff. glaucescens | AV 05-374 (GENT) | North America | KF220049 | KF220150 | KF220236 | KR364405 |
| Lactifluus aff. glaucescens | JN 2011-014 (GENT) | Vietnam | KF220104 | KF220199 | KF220273 | KR364406 |
| Lactifluus aff. glaucescens | LTH 274 (GENT) | Thailand | KR364107 | KR364238 | KR364325 | KR364457 |
| Lactifluus aff. piperatus | JN 2011-036 (GENT) | Vietnam | KF220105 | KF220200 | KF220274 | KR364454 |
| Lactifluus aff. piperatus | JN 2011-072 (GENT) | Vietnam | KF220106 | KF220201 | KF220275 | KR364455 |
| Lactifluus aff. piperatus | TENN 064342 (TENN) | North America | KR364103 | KR364234 | KR364324 | KR364456 |
| Lactifluus dwaliensis | LTH 55 (GENT) | Thailand | KF220111 | KF220204 | KF220278 | KR364386 |
| Lactifluus dwaliensis Type | KD 612 (GENT) | India | KR364042 | – | – | – |
| Lactifluus glaucescens | LGAM 2010-0132 (LGAM-AUA) | Greece | KR364105 | KR364236 | KR364280 | KR364407 |
| Lactifluus leucophaeus | LTH 182 (GENT) | Thailand | KF220059 | KF220157 | KF220243 | KR364420 |
| Lactifluus piperatus | 2001 08 19 68 (GENT) | France | KF220119 | KF241840 | KF241842 | KR364453 |
| Lactifluus roseophyllus | JN 2011-076 (GENT) | Vietnam | KF220107 | KF220202 | KF220276 | KR364464 |
| Genus Russula | ||||||
| Russula cyanoxantha | FH 12-201 (GENT) | Germany | KR364093 | KR364225 | KR364341 | KR364471 |
| Russula delica | FH 12-272 (GENT) | Belgium | KF432955 | KR364224 | KR364340 | KR364470 |
| Russula gracillima | FH 12-264 (GENT) | Germany | KR364094 | KR364226 | KR364342 | KR364472 |
| Russula khanchanjungae | AV-KD-KVP 09-106 (GENT) | India | KR364129 | JN389004 | JN375607 | JN389092 |
| Russula sp. | EDC 12-061 (GENT) | Cameroon | KR364072 | KR364201 | KR364338 | KR364468 |
| Russula sp. | EDC 12-063 (GENT) | Cameroon | KR364073 | KR364202 | KR364339 | KR364469 |
| Genus Lactarius | ||||||
| Lactarius fuliginosus | MTB 97-24 (GENT) | Sweden | JQ446111 | JQ446180 | JQ446240 | KR364392 |
| Lactarius hatsudake | FH 12-052 (GENT) | Thailand | KR364085 | KR364215 | KR364285 | KR364411 |
| Lactarius miniatescens | AV 11-177 (GENT) | Togo | KR364059 | KR364187 | KR364315 | KR364443 |
| Lactarius olympianus | ED 08-018 (GENT) | North America | KR364089 | KR364220 | KR364320 | KR364448 |
| Lactarius scrobiculatus | JN 2001-058 (GENT) | Slovakia | KF432968 | KR364219 | KR364344 | KR364474 |
| Lactarius tenellus | ADK 3598 (GENT) | Benin | KF133280 | KF133313 | KF133345 | KR364482 |
| Genus Multifurca | ||||||
| Multifurca furcata | REH 7804 (NY) | Costa Rica | DQ421995 | DQ421995 | DQ421928 | – |
| Multifurca ochricompacta | BB 02-107 (PC) | North America | DQ421984 | DQ421984 | DQ421940 | – |
| Multifurca sp. | xp2-20120922-01 (GENT) | China | KR364125 | – | – | – |
| Multifurca stenophylla | JET956 (MEL) | Australia | JX266631 | JX266635 | – | – |
| Multifurca zonaria | FH 12-009 (GENT) | Thailand | KR364083 | KR364212 | KR364365 | KR364497 |
| Outgroup | ||||||
| Amylostereum laevigatum | CBS 623.84 (CBS) | France | AY781246 | AF287843 | AY218469 | – |
| Auriscalpium vulgare | PBM 944 (WTU ) | North America | DQ911613 | DQ911614 | AY218472 | – |
| Bondarzewia montana | AFTOL 452 (DAOM) | No data | DQ200923 | DQ234539 | AY218474 | DQ256049 |
| Echinodontium tinctorium | AFTOL 455 (DAOM) | No data | AY854088 | AF393056 | AY218482 | AY864882 |
| Heterobasidion annosum | AFTOL 470 (DAOM) | No data | DQ206988 | – | AY544206 | DQ667160 |
| Stereum hirsutum | AFTOL 492 | No data | AY854063 | AF393078 | AY218520 | AY864885 |
| Vararia abortiphysa | CBS 630.81 (CBS) | France | KR364005 | KR364133 | KR364266 | – |
Morphological analyses
For each Lactifluus collection, several important or striking morphological characteristics were determined. The following characteristics, traditionally used to characterise infrageneric groups, are represented in the phylogenetic trees of each sub-genus:
fruit body type (agaricoid/pleurotoid);
presence or absence of a secondary velum;
colour reaction of the latex and/or the context when exposed to the air;
pileipellis type (Fig. 1); and
presence or absence of true cystidia, together with cystidium type (macro-, lepto- or lamprocystidia, Fig. 2).
Fig. 1.
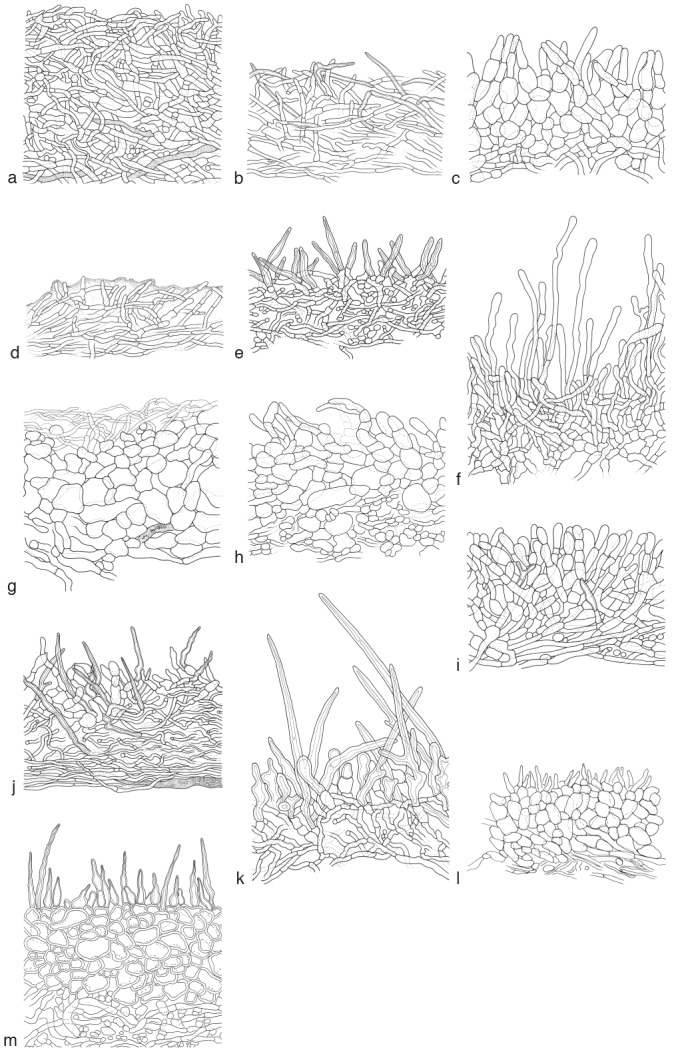
Overview of different pileipellis types found in the genus Lactifluus. a. Cutis in Lf. urens (JR 6002); b. irregular cutis in Lf. madagascariensis (BB 97-072); c. hymeniderm in Lf. roseolus (AV 94-064); d. ixotrichoderm in Lf. rufomarginatus (ADK 3011); e. lamprotrichoderm in Lf. pruinatus (BB 3248); f. trichoderm in Lf. aurantiifolius (AV 94-063); g. hyphoepithelium in Lf. piperatus (HP 8475); h. trichopalisade in Lf. xerampelinus (TS 1116); i. mixed trichopalisade in Lf. indusiatus (AV 94-122); j. mixed trichopalisade abundant thick-walled elements in Lf. sesemotani (GF 143); k. lamprotrichopalisade in Lf. heimii (AV 94-465); l. palisade in Lf. atrovelutinus (DS 06-003); m. lampropalisade in Lf. oedematopus (RW 1228). — Drawings by: a–k. A. Verbeken; l. D. Stubbe; m. K. Van de Putte.
Fig. 2.
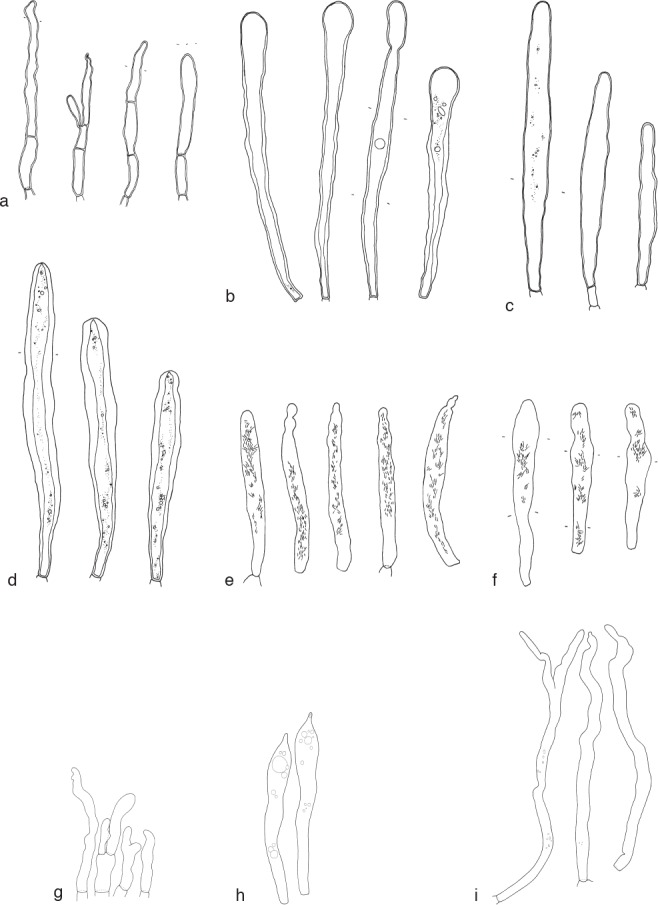
Overview of different cystidium types found in the genus Lactifluus. — a–d. Lamprocystidia: a. in Lf. armeniacus (EDC 14-501); b. in Lf. sp. nov. (AV 11-006); c. in Lf. cf. pumilus (EDC 12-066); d. in Lf. cf. volemus (REH 9320). — e–f. Macrocystidia: e. in Lf. sp. nov. (JN 2011-077); f. in Lf. roseophyllus (JN 2011-076). — g–i. Leptocystidia: g. in Lf. ruvubuensis (AV 94-599); h. in Lf. indusiatus (AV 94-122); i. in Lf. densifolius (BB 3601). — Drawings by: a–f. E. De Crop; g–i. A. Verbeken.
Other morphological characteristics were discussed depending on their importance as delimiting features.
Macromorphological characteristics of fresh material were described in daylight conditions and morphology of herbarium specimens was based on the notes of the collectors or was obtained from the original species descriptions. Micromorphological characteristics were studied on dried herbarium collections or derived from the original species descriptions. We follow Vellinga (1988) for general terminology and Verbeken & Walleyn (2010) for terminology concerning pileipellis structures. Basidiospores were measured in side view, in Melzer’s reagent. Measurements exclude ornamentations. Elements of the pileipellis and the hymenium were measured halfway the radius of the pileus in Congo-Red in L4, using an Olympus CX31 microscope.
DNA extraction, PCR amplification, sequencing and nucleotide alignments
DNA from fresh material was extracted using the CTAB extraction described in Nuytinck & Verbeken (2003), whereas DNA of dried material was extracted using the protocol of Nuytinck & Verbeken (2003) with modifications described in Van de Putte et al. (2010). Protocols for PCR amplification follow Le et al. (2007a). In order to get support for branches at and above species level, we chose genes proven to be informative across multiple phylogenetic levels within the Russulaceae (Buyck et al. 2008, Van de Putte et al. 2012):
the internal transcribed spacer region of ribosomal DNA (ITS), comprising the ITS1 and ITS2 spacer regions and the ribosomal gene 5.8S. Primers ITS-1F/ITS5 and ITS4 were used (White et al. 1990, Gardes & Bruns 1993), together with internal primers ITS2 and ITS3 (White et al. 1990) for old type specimens and poorly dried collections;
a part of the ribosomal large subunit 28S region (LSU), using primers LR0R and LR5 (Moncalvo et al. 2000);
the region between the conserved domains 6 and 7 of the second largest subunit of the RNA polymerase II (RPB2), using primers bRPB2-6F and fRPB2-7cR (Liu et al. 1999, Matheny 2005); and
the region between domains A and C of nuclear gene encoding the largest subunit of RNA polymerase II (RPB1), using primers RPB1-Ac and RPB1-Cr (Stiller & Hall 1997, Matheny et al. 2002). As the RPB1 fragment is over 1300 bp long, sequencing often failed for dried material. Based on existing RPB1 sequences of milkcap species, we constructed an internal primer, with primer sequences RPB1-F3: 5′-AGT AAR AYG RTY TGT GAG GC-3′ and RPB1-R4: 5′ - GCC TCA CAR AYC RTY TTA CT - 3′. Then, using primer pairs RPB1-Ac/RPB1-R4 and RPB1-F3/RPB1-Cr, two fragments of RPB1 were obtained and joined for alignment and phylogenetic analyses.
PCR products were sequenced using an automated ABI 3730 XL capillary sequencer (Life Technology) at Macrogen. Forward and reverse sequences were assembled into contigs and edited where needed with the SequencherTM v. 5.0 software (Gene Codes Corporation, Ann Arbor, MI, USA). Sequences were aligned using the online version of the multiple sequence alignment program MAFFT v. 7 (Katoh & Toh 2008), using the E-INS-I strategy. Trailing ends of the alignment were trimmed and alignments were manually edited when necessary in Mega 6 (Tamura et al. 2013). We choose not to exclude ambiguously aligned regions from the alignment (either manually or by a computer program), as it was shown by Nagy et al. (2012) that the deletion of gapped sites universally decreases tree resolution and branch support. Four final alignments were used:
a combined alignment of ITS+LSU sequence data;
an alignment of RPB2 sequence data;
an alignment of RPB1 sequence data; and
a combined alignment of ITS+LSU, RPB2 and RPB1 sequence data.
The alignments can be acquired from the first author and TreeBASE (S17930).
Phylogenetic analyses
Sequence data were divided into the following partitions. The ITS+LSU alignment was partitioned into partial 18S, ITS1, 5.8S, ITS2 and partial 28S. Both RPB2- and RPB1-alignments were partitioned into the intron(s) and the first, second and third codon positions of the exon. Maximum Likelihood (ML) analyses were conducted with RAxML v. 8.0.24 (Stamatakis 2014), where an ML analysis was combined with the Rapid Bootstrapping algorithm with 1 000 replicates under the GTRCAT option (Stamatakis et al. 2008). Bayesian Inference (BI) was executed with MrBayes v. 3.2.0 (Ronquist et al. 2012). Partitionfinder v. 1.1.1 (Lanfear et al. 2012) was first used to determine the model that best fits each partition, using the Bayesian information criterion (BIC), after which we evaluated the chosen models. Models found by Partitionfinder under BIC were: 18S: JC+I, ITS1: GTR+G+I, 5.8S: K80+G+I, ITS2: GTR+G+I, 28S: GTR+G+I, RPB1pos1: K80+G+I, RPB1pos2: K80+G+I, RPB1pos3: GTR+G+I, RPB1intron1: HKY+G+I, RPB1intron2: GTR+G+I, RPB1intron3: K80+G+I, RPB1intron4: GTR+G+I, RPB2pos1: K80+G+I, RPB2pos2: TVM+G+I, RPB2pos3: GTR+G+I, RPB2intron: HKY+G+I. The BIC criterion mostly favoured +G+I models. However, we chose to only add the gamma model (G) and leave the estimation of invariant sites (I) out, as several studies have shown that both parameters correlate, which may not always be favourable (Jia et al. 2014, Drummond & Bouckaert 2015). Four parallel runs, each consisting of one cold and three heated chains, were performed for 10 million generations sampling every 100th generation for the single gene trees and 20 million generations sampling every 1 000th generation for the concatenated tree. Parameter convergence for the different runs was verified in Tracer v. 1.6 (Rambaut et al. 2014) and AWTY (Nylander et al. 2008). After discarding a burn-in determined in Tracer, a majority rule consensus tree was constructed. ML and BI analyses were performed on each of the four alignments. All analyses were performed on the CIPRES Science Gateway (Miller et al. 2010).
RESULTS
Our dataset contains 213 Russulales collections, of which 189 are from the genus Lactifluus. With approximately 150 described species in Lactifluus, 80 % of the described taxa are represented in our dataset. Of the 20 % missing, most species are only known from collections too old for sequencing. The remainder are taxa from species complexes represented by at least 15 species in our dataset, for instance from Lf. subg. Gerardii and Lf. sect. Lactifluus. These complexes have been studied before and their absence in this analysis does not affect stability of the results (Stubbe et al. 2010, Van de Putte et al. 2010, 2012). Fifty-one of the described species we included have never been sequenced before and 46 of the described species are represented by their type specimen. Furthermore, we included 30 unidentified collections, of which at least 15 represent new species. PCR and sequencing success rate differed among the four genes, with 213, 195, 177 and 151 sequences obtained for ITS, LSU, RPB2 and RPB1, respectively. A total of 493 new sequences were generated for this study, the remaining were obtained from our previous studies and GenBank. ML and BI results of the three independent datasets are similar, without any supported conflicts (support: ML > 70, BI > 0.95). We therefore used the concatenated dataset, which is 5032 bp long (including gaps).
The phylogeny of the concatenated data is shown in Fig. 3. The outgroup is fully supported (ML: 100, BI: 1), as are the genera Russula (ML: 99, BI: 1), Lactarius (ML: 100, BI: 1) and Multifurca (ML: 100, BI: 1). Lactifluus is well-supported (ML: 98, BI: 1) and can be divided in four supported clades, corresponding to four subgenera: Lf. subg. Lactariopsis (ML: 89, BI: 0.97), Lf. subg. Pseudogymnocarpi (ML: 99, BI: 1), Lf. subg. Gymnocarpi (ML: 99, BI: 1) and Lf. subg. Lactifluus (ML: 99, BI: 1). Representatives of each subgenus are shown in Fig. 4 and 5. Each subgenus can be further divided into several sections, which are described below, together with their known morphological characteristics.
Fig. 3.
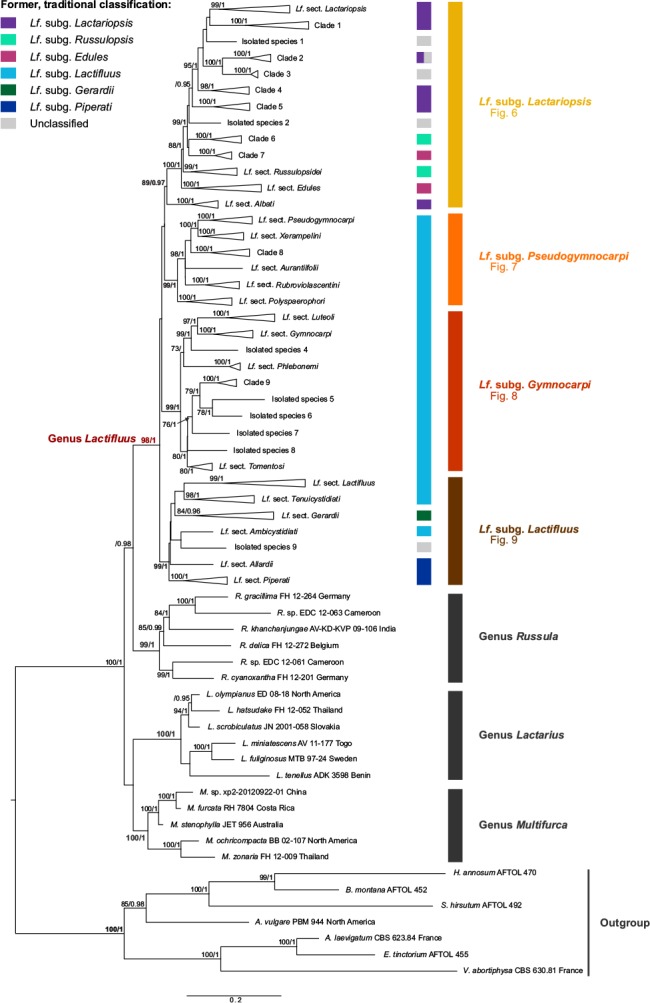
Overview Maximum Likelihood tree of the genus Lactifluus, based on concatenated ITS, LSU, RPB2 and RPB1 sequence data. The first column of colour bars represents the former, traditional classification. The second column represents the newly proposed classification. Maximum Likelihood bootstrap values > 70 and Bayesian Inference posterior probabilities > 0.95 are shown.
Fig. 4.

Basidiocarps of representative species from the different subgenera and sections within the genus Lactifluus. — a–f. Lf. subg. Lactariopsis: a. Lf. sect. Lactariopsis: Lf. sp. (EDC 14-060, De Crop E); b. Clade 3: Lf. multiceps (TH9807, Elliot T); c. Clade 5: Lf. leoninus (DS 07-462, Stubbe D); d. Lf. sect. Russulopsidei: Lf. longipes (EDC 12-049, De Crop E); e. Lf. sect. Edules: Lf. sp. nov. (EDC 12-069, De Crop E); f. Lf. sect. Albati: Lf. vellereus (Slos D). — g–i. Lf. subg. Pseudogymnocarpi: g. Lf. sect. Pseudogymnocarpi: Lf. pumilus (EDC 12-066, De Crop E); h. Lf. sect. Pseudogymnocarpi: Lf. rugatus (18.10.09, Pera U); i. Lf. sect. Xerampelini: Lf. sp. nov. (EDC 12-001, De Crop E); j. Lf. sect. Xerampelini: Lf. kigomaensis (EDC 11-159, De Crop E); k. Clade 8: Lf. armeniacus (EDC 14-501, De Crop E); l. Lf. sect. Rubroviolascentini: Lf. aff. rubroviolascens (EDC 12-051, De Crop E).
Fig. 5.

Basidiocarps of representative species from the different subgenera and sections within the genus Lactifluus. — a–f. Lf. subg. Gymnocarpi: a. Lf. sect. Luteoli: Lf. brunneoviolascens (Boerio G); b. Lf. sect. Gymnocarpi: Lf. gymnocarpus (EDC 12-047, De Crop E); c. Lf. sect. Gymnocarpi: Lf. albomembranaceus nom. prov. (EDC 12-046, De Crop E); d. Lf. sect. Phlebonemi: Lf. aff. phlebonemus (EDC 12-067, De Crop E); e. isolated species 6: Lf. brunellus (TH 7684, Henkel T); f. Lf. sect. Tomentosi: Lf. subclarkeae (RH 9223, Halling R). — g–l. Lf. subg. Lactifluus: g. Lf. sect. Lactifluus: Lf. volemus (Boerio G); h. Lf. sect. Tenuicystidiati: Lf. sp. (JN 2011-080, Nuytinck J); i. Lf. sect. Gerardii: Lf. bicolor (DS 06-229, Stubbe D); j. Lf. sect. Gerardii: Lf. sp. (EDC 14-500, De Crop E); k. Lf. sect. Allardii: Lf. allardii (C.C. 3.0, Molter D); l. Lf. sect. Piperati: Lf. aff. piperatus (JN 2011-072, Nuytinck J).
I. Lactifluus subg. Lactariopsis — Fig. 3, 4a, b, c, d, e, f, 6
Fig. 6.
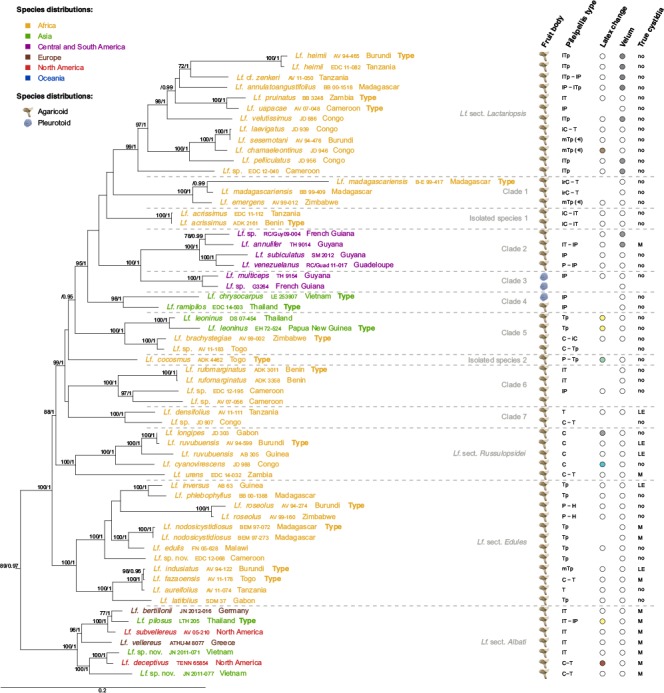
Maximum Likelihood tree of Lactifluus subg. Lactariopsis, based on concatenated ITS, LSU, RPB2 and RPB1 sequence data. Maximum Likelihood bootstrap values > 70 and Bayesian Inference posterior probabilities > 0.95 are shown. Tip labels are coloured according to species’ distributions, see figure for colour legend. Five morphological characteristics are plotted to the right of the tip labels. Fruit body type is represented by a symbol of an agaricoid or pleurotoid fungus. Pileipellis types are presented as a combination of the following abbreviations: C = cutis; H = hymeniderm; T = trichoderm; P = palisade; Tp = trichopalisade; i = ixo-; l = lampro-; ir = irregular; m = mixed; (+l) = with abundant thick-walled elements. Latex colour change is represented by coloured circles, where white circles indicate no colour change and striped circles indicate transparent latex. Velum presence is indicated by grey, whereas velum absence is indicated by white dots. Presence of true cystidia is represented by the following abbreviations: no = no true cystidia observed; M = pleuromacrocystidia present; LE = pleuroleptocystidia present. For all characteristics, blanks indicate unknown character states.
Lactifluus subg. Lactariopsis is well-supported by molecular results. The subgenus is characterised by a variety of pileipellis types, ranging from types with abundant to scarce needle-shaped thick-walled elements. In most species true pleurocystidia are absent, but pleuromacrocystidia or pleuroleptocystidia are present in some, while pleurolamprocystidia were never observed. This is the only clade in which species with secondary velum occur and colour changes of the context and/or latex are only rarely observed. The subgenus consists of eleven well-supported clades and two species on isolated branches:
– In the exclusively African Lf. sect. Lactariopsis, former representatives of Lf. sect. Lactariopsis (species with velum) and Lf. sect. Chamaeleontini (species without velum) are mixed. This section can be recognised by a combination of thick-walled elements in the pileipellis and pseudocystidia that are highly emergent (up to 50 μm in Lf. annulatoangustifolius) and broad (up to 25 μm diam in Lf. zenkeri).
– Clade 1 contains two African species: Lf. madagascariensis and Lf. emergens. They can be recognised by the combination of narrow and only slightly emergent pseudocystidia, thick-walled elements in the pileipellis and the absence of secondary velum.
– Lactifluus acrissimus, sister to the preceding two clades, is isolated on a rather long branch. Until now, this species was considered to belong to Lactarius (Van Rooij et al. 2003), but our molecular study of the type sequence shows that it belongs to Lactifluus. It is characterised by creamy white cap colours, an ixocutis to ixotrichoderm as pileipellis and a burning acrid taste.
– Clade 2 contains several agaricoid South American species. Species from this clade all have thick-walled elements in the pileipellis and comprise all known South American taxa with secondary velum on the stipe, as an annulus, and on the pileus margin.
– Clade 3 contains two pleurotoid species from South America, of which Lf. multiceps can be recognised by its orange cap colours, a lampropalisade and the absence of secondary velum and true cystidia.
– Clade 4 contains two Asian species: the small pleurotoid Lf. chrysocarpus, which was already mentioned to belong to Lf. subg. Lactariopsis in the study of Morozova et al. (2013), and the recently described Lf. ramipilosus (Li et al. 2016). Both are characterised by a lampropalisade and the absence of a secondary velum.
– Clade 5 is composed of African and Asian species. They all have pseudocystidia that are highly emergent (up to 40 μm in Lf. brachystegiae) and thick (up to 18 μm diam in Lf. brachystegiae), a cutis to trichopalisade as pileipellis and no secondary velum or true cystidia.
– Lactifluus cocosmus is another species isolated on a rather long branch. As previously mentioned by Van de Putte et al. (2009), it has a deviating morphology, with latex turning greenish and a distinct coconut odour. There are no close relatives known.
– Clade 6 contains three African agaricoid species, two of which are possible new taxa from Cameroon. Lactifluus rufomarginatus is characterised by an ixopalisade as pileipellis, which is rare in the genus.
– Clade 7 consists of two African representatives. Both have a cutis to a trichopalisade as pileipellis and Lf. densifolius is also characterised by the presence of pleuroleptocystidia.
– Species from Lf. sect. Russulopsidei are characterised by brown-red colours in cap and stipe, a cutis as pileipellis, the presence of dermatocystidia and the absence of a velum. Several species also have true pleurocystidia.
– Lactifluus sect. Edules corresponds to the original Lf. subg. Edules. This entirely African clade is characterised by agaricoid species with firm basidiocarps, yellowish to greyish orange colours, a trichoderm to (tricho) palisade as pileipellis and the lack of conspicuous thick-walled terminal elements in the pileipellis. The smallest representative, Lf. roseolus, has a slightly deviating morphology with its small basidiocarps, but its microscopic characteristics perfectly fit in this section. Unexpectedly, a former representative of Lf. sect. Chamaeleontini, Lf. indusiatus, also belongs to this clade.
– Lactifluus sect. Albati has Northern hemisphere representatives only. They are characterised by large, white and mostly velutinous agaricoid basidiocarps, a lamprotrichoderm as pileipellis and/or stipitipellis composed of thick-walled hairs even up to 400 μm in Lf. vellereus and slightly to clearly moniliform pleuromacrocystidia.
II. Lactifluus subg.Pseudogymnocarpi — Fig. 3, 4g, h, i, j, k, l, 7
Fig. 7.
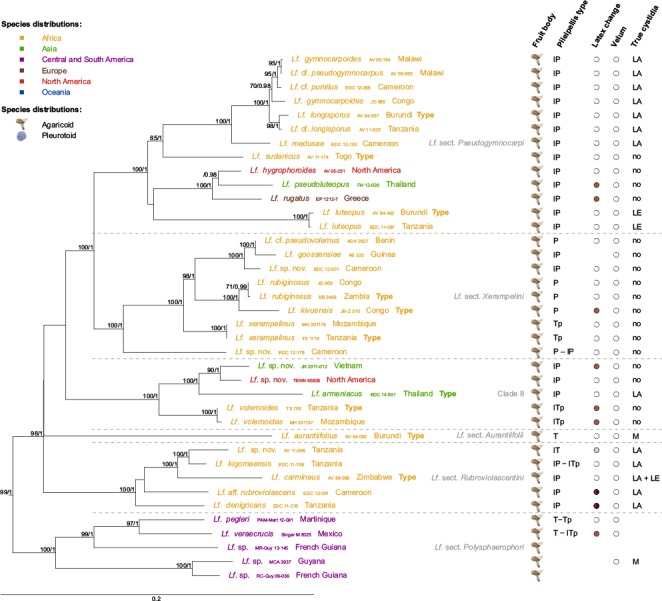
Maximum Likelihood tree of Lactifluus subg. Pseudogymnocarpi, based on concatenated ITS, LSU, RPB2 and RPB1 sequence data. Maximum Likelihood bootstrap values > 70 and Bayesian Inference posterior probabilities > 0.95 are shown. Tip labels are coloured according to species’ distributions, see figure for colour legend. Five morphological characteristics are plotted to the right of the tip labels. Fruit body type is represented by a symbol of an agaricoid or pleurotoid fungus. Pileipellis types are presented as a combination of the following abbreviations: T = trichoderm; P = palisade; Tp = trichopalisade; l = lampro-. Latex colour change is represented by coloured circles, where white circles indicate no colour change and striped circles indicate transparent latex. Velum presence is indicated by grey, whereas velum absence is indicated by white dots. Presence of true cystidia is represented by the following abbreviations: no = no true cystidia observed; M = pleuromacrocystidia present; LE = pleuroleptocystidia present; LA = pleurolamprocystidia present. For all characteristics, blanks indicate unknown character states.
Species of Lactifluus subg. Pseudogymnocarpi are all agaricoid species characterised by yellow, orange to reddish brown caps and a trichoderm to (lampro) (tricho) palisade as pileipellis. In some species, true pleurocystidia are absent, while others have pleurolamprocystidia or pleuromacrocystidia. Some species show striking colour reactions of the latex, but most species do not. The subgenus consists of five well-supported clades and one isolated species:
– Lactifluus sect. Pseudogymnocarpi is represented by several African species and a subclade with one North American, one Asian and one European species. This section is characterised by a lampropalisade as pileipellis and some species have pleurolampro- or pleuroleptocystidia in their hymenium.
– Lactifluus sect. Xerampelini is an exclusively African clade. Species have yellowish orange to reddish brown cap colours. They have palisade-like structures as pileipellis, and only some of them have thick-walled terminal elements. They lack true pleurocystidia and spores generally have low ornamentation (usually not higher than 0.2 μm) and are verrucose or have a more or less complete reticulum.
– Clade 8 has African, Asian and North American representatives, of which several are undescribed. All representatives have palisade-like structures with thick-walled elements as pileipellis and lack true pleurocystidia, except Lf. armeniacus which has pleuromacrocystidia.
– Lactifluus sect. Aurantiifolii contains the single, isolated species Lf. aurantiifolius. As noted by Verbeken & Walleyn (2010), this species is characterised by a combination of several unique characters: bright orange lamellae, a white and fimbriate lamellar edge, a zonate and highly pruinose pileus and a chambered, tapering stipe.
–Lactifluus sect. Rubroviolascentini is an exclusively African clade. It unites species with latex that changes from cream to red and finally black, together with species that lack these colour reactions. All are characterised by pleurolamprocystidia and Lf. carmineus even has both pleurolampro- and pleuroleptocystidia.
– Lactifluus sect. Polysphaerophori only contains Central and South American species. Collections or their morphological descriptions were not available for most species so general characteristics are thus hard to define.
III. Lactifluus subg. Gymnocarpi — Fig. 3, 5a, b, c, d, e, f, 8
Fig. 8.
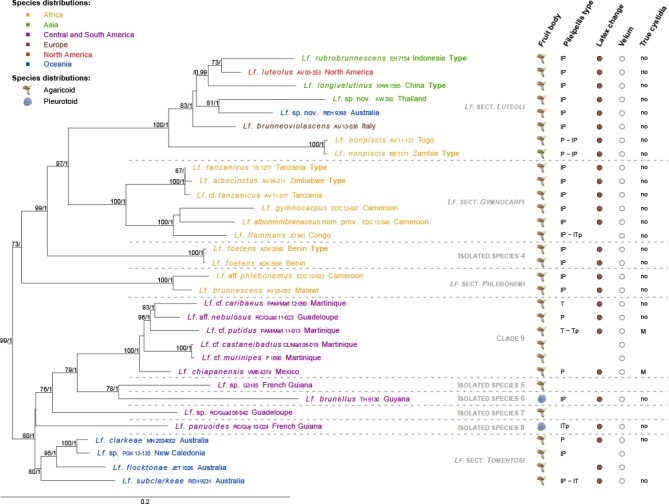
Maximum Likelihood tree of Lactifluus subg. Gymnocarpi, based on concatenated ITS, LSU, RPB2 and RPB1 sequence data. Maximum Likelihood bootstrap values > 70 and Bayesian Inference posterior probabilities > 0.95 are shown. Tip labels are coloured according to species’ distributions, see figure for colour legend. Five morphological characteristics are plotted to the right of the tip labels. Fruit body type is represented by a symbol of an agaricoid or pleurotoid fungus. Pileipellis types are presented as a combination of the following abbreviations: T = trichoderm; P = palisade; Tp = trichopalisade; l = lampro-. Latex colour change is represented by coloured circles, where white circles indicate no colour change and striped circles indicate transparent latex. Velum presence is indicated by grey, whereas velum absence is indicated by white dots. Presence of true cystidia is represented by the following abbreviations: no = no true cystidia observed; M = pleuromacrocystidia present. For all characteristics, blanks indicate unknown character states.
Lactifluus subg. Gymnocarpi can be recognised by a combination of a lampropalisade as pileipellis, the absence of true pleurolamprocystidia (with discrete pleuromacrocystidia rarely present) and a brownish colour reaction of the latex and/or the context when exposed to air. The subgenus consists of five supported clades and five isolated species:
– Typical for Lf. sect. Luteoli, which consists of species from all continents except South America, are the capitate elements in the pileipellis and/or marginal cells. Verbeken & Walleyn (2010) already suggested that species with capitate terminal pileipellis elements might form a natural group. Lactifluus brunneoviolascens, the European representative, is often confused with the similar North American Lf. luteolus. Our study indicates that the North American species is different from the European one, which means that Lf. luteolus is an incorrect name for the European taxon.
– Lactifluus sect. Gymnocarpi has only African representatives. They have (slightly) thick-walled and sometimes strongly emergent marginal cells (cheilolamprocystidia) and cylindrical or irregularly shaped and often branched, thick-walled hairs in the pileipellis.
– Lactifluus foetens is isolated on a branch sister to the preceding two sections. Macroscopically, it resembles the recently described species Lf. albomembranaceus nom. prov. (EDC 12-046) of Lf. sect. Gymnocarpi, but their microscopic characteristics do not correspond. The pileipellis of Lf. foetens, for example, is a lampropalisade with tufts of long, slender and regular subcylindric hairs, while the pileipellis of the undescribed species is a lampropalisade with a layer of shorter, broad and irregular subcylindric hairs.
– Lactifluus sect. Phlebonemi contains two tropical African species. They seem to have slightly different latex characteristics compared to the other species of Lf. subg. Gymnocarpi. Their latex quickly turns brownish in contact with the lamellae or the context, as well as when isolated from the flesh. Furthermore, the latex is rather whey-like and does not colour evenly.
– The remaining species form one large clade, containing several subclades with species from Oceania, Central and South America. Within this species-rich lineage, clade 9 entirely consists of Central and South American taxa. Molecularly it is well-supported, but unfortunately, thorough morphological descriptions are lacking for most of these collections. Basal to the former clade, there are four isolated species on separate branches from Central and South America: Lf. brunellus, Lf. panuoides and two undescribed species (G3185 and RC/Guad 08-042). Both Lf. panuoides and Lf. brunellus have a pleurotoid habitat, the other two specimens are agaricoid. The Oceanian species group in Lf. sect. Tomentosi. This section is supported in both concatenated analyses, but does not get high support in the individual gene phylogenies. It includes R. flocktonae, originally placed in Russula (Cleland & Cheel 1919). Singer (1942) noted that it could be Lactarius clarkeae and Lebel et al. (2013) also indicated that it belongs to Lactifluus. In our analyses it is sister to Lf. clarkeae and we will recombine this in Lactifluus.
IV. Lactifluus subg. Lactifluus — Fig. 3, 5g, h, i, j, k, l, 9
Fig. 9.
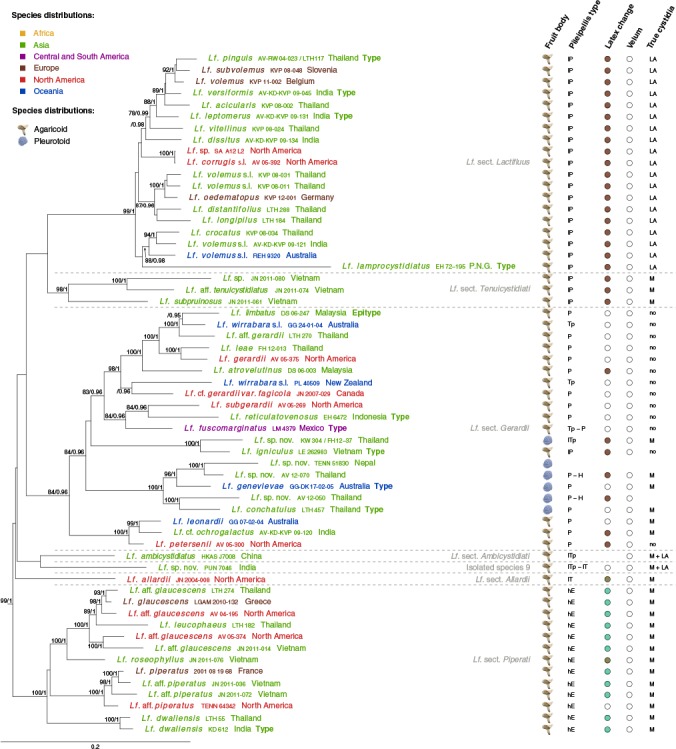
Maximum Likelihood tree of Lactifluus subg. Lactifluus, based on concatenated ITS, LSU, RPB2 and RPB1 sequence data. Maximum Likelihood bootstrap values > 70 and Bayesian Inference posterior probabilities > 0.95 are shown. Tip labels are coloured according to species’ distributions, see figure for colour legend. Five morphological characteristics are plotted to the right of the tip labels. Fruit body type is represented by a symbol of an agaricoid or pleurotoid fungus. Pileipellis types are presented as a combination of the following abbreviations: H = hymeniderm; T = trichoderm; hE = hyphoepithelium; P = palisade; Tp = trichopalisade; l = lampro-. Latex colour change is represented by coloured circles, where white circles indicate no colour change and striped circles indicate transparent latex. Velum presence is indicated by grey, whereas velum absence is indicated by white dots. Presence of true cystidia is represented by the following abbreviations: no = no true cystidia observed; M = pleuromacrocystidia present; LA = pleurolamprocystidia present. For all characteristics, blanks indicate unknown character states. In the tip labels, P.N.G. stands for Papua New Guinea.
Lactifluus subg. Lactifluus is characterised by a range of pileipellis types, from a hyphoepithelium over a palisade to a lampropalisade. In some sections, true pleurocystidia are absent, while in others pleuromacrocystidia and/or pleurolamprocystidia are found. Most species are agaricoid, only Lf. sect. Gerardii has several pleurotoid representatives. For some sections, the colour reaction of the context and/or the latex upon contact with air is an important characteristic. The subgenus contains species from Asia, Europe, North America and Oceania and consists of six separate clades, all molecularly and morphologically well-supported. These clades correspond well to current classifications and we recognize them here at section level: Lf. sect. Allardii, Lf. sect. Ambicystidiati, Lf. sect. Gerardii, Lf. sect. Lactifluus, Lf. sect. Piperati and Lf. sect. Tenuicystidiati. Lactifluus sect. Gerardii is equivalent to Lf. subg. Gerardii described in the introduction, but to limit the number of subgenera in Lactifluus, we decided to treat it as section. The other five sections correspond to those described in the introduction.
TAXONOMIC PART
Genus
Genus Lactifluus (Pers.) Roussel, Fl. Calvados, Ed. 2: 66. 1806
Basionym. Agaricus sect. Lactifluus Pers., Syn. Meth. Fung.: 429. 1801.
= Pleurogala Redhead & Norvell, Mycotaxon 48: 377. 1993.
≡ Lactarius sect. Panuoidei Singer, Kew Bull. 7: 301. 1952.
Type (automatic). Agaricus lactifluus L., Sp. Pl.: 1172. 1753 (= Lactifluus volemus (Fr.: Fr.) Kuntze).
Subgenera
Lactifluus subg. Gymnocarpi (R. Heim ex Verbeken) De Crop, comb. nov. — MycoBank MB814217
Basionym. Lactarius sect. Gymnocarpi R. Heim ex Verbeken, Mycotaxon 66: 374. 1998.
Type. Lactarius gymnocarpus R. Heim ex Singer, Pap. Michigan Acad. Sci. 32: 107. 1946 (≡ Lactifluus gymnocarpus (R. Heim ex Singer) Verbeken).
Lactifluus subg. Lactariopsis (Henn.) Verbeken, Mycotaxon 118: 449. 2011
Basionym. Lactariopsis Henn., Bot. Jahrb. Syst. 30: 51. 1901.
≡ Lactarius subg. Lactariopsis (Henn.) R. Heim, Prodr. Fl. Mycologique Madagascar 1: 36. 1938.
= Lactarius sect. Edules Verbeken, Belg. J. Bot. 132: 176. 2000 (1999).
≡ Lactifluus subg. Edules (Verbeken) Verbeken, Mycotaxon 118: 448. 2011.
= Lactarius subg. Russulopsis Verbeken, Mycotaxon 77: 439. 2001.
≡ Lactifluus subg. Russulopsis (Verbeken) Verbeken, Mycotaxon 118: 452. 2011.
Type. Lactariopsis zenkeri Henn., Bot. Jahrb. Syst. 30: 51. 1902 (1901) (≡ Lactifluus zenkeri (Henn.) Verbeken).
Lactifluus subg. Lactifluus
≡ Lactarius subg. Lactiflui (Burl.) Hesler & A.H. Sm., N. Amer. Sp. Lactarius: 158. 1979.
= Lactifluus subg. Gerardii (A.H. Sm. & Hesler) Stubbe, Mycotaxon 119: 484. 2012.
≡ Lactarius subg. Gerardii (A.H. Sm. & Hesler) Stubbe, Fungal Biol. 114: 280. 2010.
≡ Lactarius ser. Gerardii A.H. Sm. & Hesler, Brittonia 14: 378. 1962.
= Lactifluus subg. Piperati Verbeken, Mycotaxon 120: 449. 2012.
Type (automatic). Agaricus lactifluus L., Sp. Pl.: 1172. 1753 (= Lactifluus volemus (Fr.: Fr.) Kuntze).
Lactifluus subg. Pseudogymnocarpi (Verbeken) De Crop, comb. nov. — MycoBank MB814193
Basionym. Lactarius sect. Pseudogymnocarpi Verbeken, Mycotaxon 66: 376. 1998.
≡ Lactifluus sect. Pseudogymnocarpi (Verbeken) Verbeken, Mycotaxon 120: 447. 2012.
≡ Lactarius sect. Rugati Verbeken, Mycotaxon 66: 372. 1998, nom illegit. (Art. 52.1).
≡ Lactarius subsect. Rugati Pacioni & Lalli, Mycotaxon 44: 190. 1998, nom illegit. (Art. 52.1).
Type. Lactarius gymnocarpoides Verbeken, Mycotaxon 55: 530. 1995 (≡ Lactifluus gymnocarpoides (Verbeken) Verbeken).
Sections
Within Lactifluus subg. Gymnocarpi:
Lactifluus sect. Luteoli (Pacioni & Lalli) Verbeken, comb. nov. — MycoBank MB814194
Basionym. Lactarius subsect. Luteoli Pacioni & Lalli, Mycotaxon 44: 190. 1992.
≡ Lactarius sect. Luteoli (Pacioni & Lalli) Pierotti, Boll. Gruppo Micol. Bres. 48: 54. 2007.
Type. Lactarius luteolus Peck, Bull. Torrey Bot. Club 23: 412. 1896 (≡ Lactifluus luteolus (Peck) Verbeken).
Lactifluus sect. Gymnocarpi (R. Heim ex Verbeken) De Crop, comb. nov. — MycoBank MB814195
Basionym. Lactarius sect. Gymnocarpi R. Heim ex Verbeken, Mycotaxon 66: 374. 1998.
Type. Lactarius gymnocarpus R. Heim ex Singer, Pap. Michigan Acad. Sci. 32: 107. 1946 (≡ Lactifluus gymnocarpus (R. Heim ex Singer) Verbeken).
Lactifluus sect.Phlebonemi (R. Heim ex Verbeken) Verbeken, Mycotaxon 120: 446. 2012
Basionym. Lactarius sect. Phlebonemi R. Heim ex Verbeken, Mycotaxon 66: 378. 1998.
Type. Lactarius phlebonemus R. Heim & Gooss.-Font., Bull. Jard. Bot. État 25: 38. 1955 (≡ Lactifluus phlebonemus (R. Heim & Gooss.-Font.) Verbeken).
Lactifluus sect.Tomentosi (McNabb) Verbeken, Mycotaxon 120: 448. 2012
Basionym. Lactarius sect. Tomentosi McNabb, New Zealand J. Bot. 9: 59. 1971.
Type. Lactarius clarkeae Cleland, Trans. & Proc. Roy. Soc. South Australia 51: 302. 1927 (as clarkei) (≡ Lactifluus clarkeae (Cleland) Verbeken).
Within Lactifluus subg. Lactariopsis:
Lactifluus sect. Albati (Bataille) Verbeken, Mycotaxon 118: 451. 2011
Basionym. Lactarius (unranked) Albati Bataille, Fl. Monogr. Astéro.: 35. 1908.
≡ Lactarius sect. Albati (Bataille) Singer, Ann. Mycol. 40: 109. 1942.
Type. Agaricus vellereus Fr., Syst. Mycol. 1: 76. 1821: Fr., loc. cit. (≡ Lactifluus vellereus (Fr.: Fr.) Kuntze).
Lactifluus sect. Edules (Verbeken) Verbeken, comb. nov. — MycoBank MB814197
Basionym. Lactarius sect. Edules Verbeken, Belg. J. Bot. 132: 176. 2000 (1999).
Type. Lactarius edulis Verbeken & Buyck, Champ. Comest. Ouest Burundi: 103. 1994. (≡ Lactifluus edulis (Verbeken & Buyck) Buyck).
Lactifluus sect. Lactariopsis Verbeken, Mycotaxon 118: 450. 2011
≡ Lactarius sect. Lactariopsis (Henn.) Singer, Ann Mycol. 40: 111. 1942.
≡ Lactarius sect. Lactariopsidei Singer, Sydowia 15: 83. 1962.
≡ Lactarius sect. Chamaeleontini Verbeken, Mycotaxon 66: 393. 1998.
Type. Lactariopsis zenkeri Henn., Bot. Jahrb. Syst. 30: 51. 1902 (1901) (≡ Lactifluus zenkeri (Henn.) Verbeken).
Lactifluus sect. Russulopsidei (Verbeken) Verbeken, Mycotaxon 118: 452. 2011
Basionym. Lactarius sect. Russulopsidei Verbeken, Mycotaxon 77: 440. 2001.
Type. Lactarius ruvubuensis Verbeken, Bull. Jard. Bot. Natl. Belg. 65: 208. 1996 (≡ Lactifluus ruvubuensis (Verbeken) Verbeken).
Within Lactifluus subg. Lactifluus:
Lactifluus sect. Lactifluus
Type (automatic). Agaricus lactifluus L., Sp. Pl.: 1172. 1753 (= Lactifluus volemus (Fr.: Fr.) Kuntze).
Lactifluus sect. Gerardii (A.H. Sm. & Hesler) Stubbe, comb. nov. — MycoBank MB814198
Basionym. Lactarius ser. Gerardii A.H. Sm. & Hesler, Brittonia 14: 378. 1962.
Type. Lactarius gerardii Peck, Bull. Buffalo Soc. Nat. Sci. 1: 57. 1873 (as L. ‘geradii’). (≡ Lactifluus gerardii (Peck) Kuntze).
Lactifluus sect. Piperati (Fr.) Verbeken, Mycotaxon 120: 449. 2012
Basionym. Agaricus sect. Piperati Fr., Syst. Mycol. 1: 73. 1821.
≡ Lactarius sect. Piperati (Fr.: Fr.) Fr., Epicr. Syst. Mycol.: 338. 1838.
Type. Agaricus piperatus L., Sp. Pl.: 1173. 1753: Fr., Syst. Mycol. 1: 76. 1821 (≡ Lactifluus piperatus (L.: Fr.) Verbeken).
Lactifluus sect. Allardii (Hesler & A.H. Sm.) De Crop, Mycotaxon 120: 450. 2012
Basionym. Lactarius sect. Allardii Hesler & A.H. Sm., N. Amer. Sp. Lactarius: 207. 1979.
Type. Lactarius allardii Coker, J. Elisha Mitchell Sci. Soc. 34: 12. 1918 (≡ Lactifluus allardii (Coker) De Crop).
Lactifluus sect. Tenuicystidiati X.H. Wang & Verbeken, Mycologia 107, 5: 954. 2015
Type. Lactarius tenuicystidiatus X.H. Wang & Verbeken, Nova Hedwigia 83, 1–2: 173. 2006 (≡ Lactifluus tenuicystidiatus (X.H. Wang & Verbeken) X.H. Wang).
Lactifluus sect. Ambicystidiati X.H. Wang, Mycologia 107, 5: 954. 2015
Type. Lactifluus ambicystidiatus X.H. Wang, Mycologia 107, 5: 948. 2015.
Within Lactifluus subg. Pseudogymnocarpi:
Lactifluus sect. Aurantiifolii (Verbeken) Verbeken, Mycotaxon 120: 450. 2012
Basionym. Lactarius sect. Aurantiifolii Verbeken, Mycotaxon 77: 441. 2001.
Type. Lactarius aurantiifolius Verbeken, Bull. Jard. Bot. Natl. Belg. 65: 197. 1996 (≡ Lactifluus aurantiifolius (Verbeken) Verbeken).
Lactifluus sect. Polysphaerophori (Singer) Verbeken, Mycotaxon 120: 445. 2012
Basionym. Lactarius sect. Polysphaerophori Singer, Beih. Sydowia 7: 106. 1973.
Type. Lactarius veraecrucis Singer, Beih. Sydowia 7: 104. 1973 (≡ Lactifluus veraecrucis (Singer) Verbeken).
Lactifluus sect. Pseudogymnocarpi (Verbeken) Verbeken, Mycotaxon 120: 447. 2012
Basionym. Lactarius sect. Pseudogymnocarpi Verbeken, Mycotaxon 66: 376. 1998.
= Lactarius sect. Rugati Verbeken, Mycotaxon 66: 372. 1998, nom. illegit. (Art. 52.1).
Type. Lactarius gymnocarpoides Verbeken, Mycotaxon 55: 530. 1995 (≡ Lactifluus gymnocarpoides (Verbeken) Verbeken).
Lactifluus sect. Rubroviolascentini (Singer) Verbeken, Mycotaxon 120: 447. 2012
Basionym. Lactarius subsect. Rubroviolascentini Singer, Ann. Mycol. 40: 114. 1942.
≡ Lactarius sect. Rubroviolascentini (Singer) Verbeken, Mycotaxon 66: 380. 1998, as ‘Rubroviolascentes’.
Type. Lactarius rubroviolascens R. Heim, Candollea 7: 377. 1938 (≡ Lactifluus rubroviolascens (R. Heim) Verbeken).
Lactifluus sect. Xerampelini De Crop, sect. nov. — MycoBank MB814199
Pileus medium to large sized, firm; pellis mat, dry, with yellowish orange, red and reddish brown colours. Lamellae moderately spaced to very distant, thick, whitish, yellowish to orange; edge concolorous. Stipe central, cylindrical, firm, dry, more or less concolorous with pileus. Context white, unchanging, firm; taste mild. Latex abundant, white to watery, unchanging, sometimes drying brownish grey. Spores ellipsoid, sometimes elongate to strongly elongate, verrucose or with a more or less complete reticulum, generally low ornamented, usually not higher than 0.2 μm; plage sometimes with central amyloid spot. True pleurocystidia absent. Pileipellis a lampropalisade to palisade or trichopalisade.
Type. Lactarius xerampelinus Karhula & Verbeken, Karstenia 38, 2: 59. 1998 (≡ Lactifluus xerampelinus (Karhula & Verbeken) Verbeken).
New combinations at species level
Lactifluus acrissimus (Verbeken & Van Rooij) Nuytinck, comb. nov. — MycoBank MB814200
Basionym. Lactarius acrissimus Verbeken & Van Rooij, Nova Hedwigia 77: 225. 2003.
Lactifluus brunellus (S.L. Mill., Aime & T.W. Henkel) De Crop, comb. nov. — MycoBank MB814201
Basionym. Lactarius brunellus S.L. Mill., Aime & T.W. Henkel, Mycologia 94, 3: 546. 2002.
Lactifluus castaneibadius (Pegler) De Crop, comb. nov. — MycoBank MB814202
Basionym. Lactarius castaneibadius Pegler, Kew Bull. 33, 4: 622. 1979.
Lactifluus chiapanensis (Montoya, Bandala & Guzmán) De Crop, comb. nov. — Mycobank MB814203
Basionym. Lactarius chiapanensis Montoya, Bandala & Guzmán, Mycotaxon 57: 412. 1996.
Lactifluus flocktonae (Cleland & Cheel) T. Lebel, comb. nov. — MycoBank MB814204
Basionym. Russula flocktonae Cleland & Cheel, Trans. & Proc. Roy. Soc. South Australia 43: 274. 1919.
Lactifluus multiceps (S.L. Mill., Aime & T.W. Henkel) De Crop, comb. nov. — Mycobank MB814205
Basionym. Lactarius multiceps S.L. Mill., Aime & T.W. Henkel, Mycologia 94, 3: 549. 2002.
Lactifluus murinipes (Pegler) De Crop, comb. nov. — MycoBank MB814206
Basionym. Lactarius murinipes Pegler, Kew Bull. 33, 4: 623. 1979.
Lactifluus nebulosus (Pegler) De Crop, comb. nov. — MycoBank MB814207
Basionym. Lactarius nebulosus Pegler, Kew Bull. 33: 610. 1979.
Lactifluus panuoides (Singer) De Crop, comb. nov. — MycoBank MB814208
Basionym. Lactarius panuoides Singer, Kew Bull. 7: 300. 1952.
Lactifluus rufomarginatus (Verbeken & Van Rooij) De Crop, comb. nov. — MycoBank MB814209
Basionym. Lactarius rufomarginatus Verbeken & Van Rooij, Nova Hedwigia 77, 1: 235. 2003.
Lactifluus uapacae (Verbeken & Stubbe) De Crop, comb. nov. — MycoBank MB814210
Basionym. Lactarius uapacae Verbeken & Stubbe, Cryptog. Mycol. 29, 2: 140. 2008.
Lactifluus venezuelanus (Dennis) De Crop, comb. nov. — MycoBank MB814211
Basionym. Lactarius venezuelanus Dennis, Kew Bull., Addit. Ser. 3: 467. 1970.
DISCUSSION
Translation of the phylogeny in a new infrageneric classification
In this study, we attempted to resolve the infrageneric classification of the genus Lactifluus. Molecular results support four major clades, which we classify as subgenera, and within these subgenera, several sections can be delimited. Not all our results are congruent with the former infrageneric classification of Lactifluus (Fig. 3), so we provide an overview of the nomenclatural changes evoked by these new results (Taxonomic Part). Most of the traditional subgenera are rejected; only Lf. subg. Lactariopsis and Lf. subg. Lactifluus are retained but amended. Two new subgenera are proposed here: Lf. subg. Gymnocarpi and Lf. subg. Pseudogymnocarpi. All four subgenera are supported in the concatenated and the individual gene phylogenies, with one exception: the RPB1 phylogeny does not support the inclusion of Lf. sect. Albati in Lf. subg. Lactariopsis. For now, we decided to include the section in Lf. subg. Lactariopsis, as the inclusion is supported in the other individual gene phylogenies and in the concatenated phylogeny. We also preferred to define the largest supported subgenera with an evenly balanced species diversity. The relationships between the subgenera are not yet fully resolved based on our phylogenetic results. To fully understand the relationships between the subgenera, more genes need to be sequenced. Several traditional sections are confirmed in their traditional delimitation (Lf. sect. Albati, Lf. sect. Allardii, Lf. sect. Ambicystidiati, Lf. sect. Aurantiifolii, Lf. sect. Edules, Lf. sect. Gerardii, Lf. sect. Lactifluus, Lf. sect. Piperati, Lf. sect. Russulopsidei and Lf. sect. Tenuicystidiati), others are polyphyletic and either synonymised (Lf. sect. Chamaeleontini and Lf. sect. Rugati) or amended (Lf. sect. Lactariopsis, Lf. sect. Luteoli, Lf. sect. Phlebonemi, Lf. sect. Polysphaerophori, Lf. sect. Pseudogymnocarpi, Lf. sect. Rubroviolascentini, Lf. sect. Tomentosi). Our analyses show ten additional clades which we suspect may represent new sections. In the present work, we only aim to assign new sections to clades that are fully supported and characterised by several synapomorphic features. The African Lf. sect. Xerampelini is newly described, as it is clearly demarked by its yellowish orange to reddish brown cap colours, a (lampro)palisade as pileipellis, the absence of true pleurocystidia and spores with low ornamentation, usually not higher than 0.2 μm, that are verrucose or forming a more or less complete reticulum. For the remaining clades we do not yet propose infrasubgeneric ranks because a more thorough sampling and a thorough search for potential synapomorphies is necessary for this to be possible. We demonstrate the existence of at least 17 undescribed species spread across the four subgenera. This supports the hypothesis that Lactifluus is a species-rich genus where the diversity has not yet been adequately characterised. The new species that are phylogenetically characterised here will be described in future publications.
Conclusions at generic level
Our molecular results support the monophyly of Lactifluus, together with monophyly of Lactarius, Russula and Multifurca. Previous analyses have shown however that this support at genus level strongly depends on outgroup choice (De Crop et al. unpubl. res.). Our phylogenies are rooted with the outgroup used in Buyck et al. (2008), with the addition of Heterobasidion annosum and the exclusion of Peniophora nuda, Albatrellus skamanius and Gloeocystidiellum porosum. Depending on the composition of the outgroup taxa, one or more of the Russulaceae genera receives less support. Further research within the order Russulales may point to better candidates as outgroup taxa for the Russulaceae. Additionally, to draw conclusions concerning the relationships between the Russulaceae-genera, the non-agaricoid genera also need to be taken into account. These are currently poorly sampled, but will be crucial to make conclusions at the generic level.
Evaluation of morphological characters
Lactifluus exhibits considerable morphological variation, with cap diameters varying from a few millimetres to more than 20 cm, agaricoid or pleurotoid fruit body types, more than ten different types of pileipellis, striking colour changes of the latex and/or context, different types of true cystidia and/or pseudocystidia, different habitats and ectomycorrhizal hosts.
In the morphological part of our study, we focus on five characteristics, which are putatively informative at the infrageneric level:
General habitus
The first characteristic is the general habitus of the basidiocarp. The majority of the studied Lactifluus species is agaricoid, only a minority is pleurotoid. So far, no sequestrate species are known, although more extensive explorations, targeting sequestrate fungi, might reveal sequestrate Lactifluus species. We confirm the results of previous studies (Miller et al. 2012, Morozova et al. 2013) which state that the pleurotoid habitus has multiple origins, since pleurotoid species occur in seven different clades in three different subgenera. Consequently, this characteristic is not informative at infrageneric level within Lactifluus, although it had previously been used to separate the obsolete genus Pleurogala (Redhead & Norvell 1993).
Presence or absence of a secondary velum
The second characteristic is the presence or absence of a secondary velum. This feature was used by Hennings (1902) as the basis for the genus Lactariopsis (including one species, Lf. zenkeri). Its importance was diminished by the definition of L. subg. Lactariopsis (including Lf. annulatoangustifolius) by Heim (1938) and later, L. sect. Lactariopsidei (including neotropical species Lf. neotropicus and Lf. annulifer) by Singer (1942, 1961) and Singer et al. (1983). As suggested by several other authors (Verbeken 1998b, Buyck et al. 2007, 2008, Verbeken & Walleyn 2010), this striking characteristic occurs in at least two clades and therefore cannot be used to delimit clades. Nevertheless, this character is phylogenetically informative, since all species with a distinct secondary velum are found within Lf. subg. Lactariopsis. Species with a distinct ring and velum at the pileus margin are only known from Africa and South America. Apart from species with a distinct velum, there are some African species, such as Lf. laevigatus and Lf. indusiatus that give the impression of a velum at the pileus margin. However, the feature is not as distinct as in Lf. heimii or Lf. velutissimus and these species never develop an annulus on the stipe. Further research is needed to determine whether these really are velar remnants. Anyhow, this feature is not informative at section level since it occurs in several clades within Lf. subg. Lactariopsis.
Colour reaction of the latex and/or the context
The third characteristic is the colour reaction of the latex and/or the context when exposed to the air. Lactifluus species show a wide variety of colour changes. These changes are informative and can be used together with other characteristics to distinguish some groups. For example, in both Lf. subg. Gymnocarpi and Lf. sect. Lactifluus there are brownish colour changes of the latex and/or the context when they are exposed to air. In other groups, these changes only occur in some species, which makes the feature uninformative. For example, the beige latex of Lf. rubroviolascens and Lf. denigricans first turns bright red and later turns blackish when exposed to air, but the other species in Lf. sect. Rubroviolascentini lack these striking colour changes.
Pileipellis type
The fourth characteristic is the pileipellis type. Several studies (Bon 1983, Heilmann-Clausen et al. 1998, Verbeken 1998a, Verbeken & Walleyn 2010) have mentioned this as one of the most important characteristics to delineate sections and subgenera within Lactifluus, as well as in Lactarius. Our study confirms this, with the restriction that the pileipellis type can only be used within some subgenera. In Lf. subg. Pseudogymnocarpi for instance, the majority of species has a lampropalisade, which makes it difficult to use the feature within the subgenus.
Presence or absence of true pleurocystidia
The fifth characteristic is the presence or absence of true pleurocystidia, together with cystidium type (macro-, lepto- or lamprocystidia). Again, this characteristic can be used to delimit some sections in combination with other characteristics. In e.g. Lf. sect. Lactifluus, the presence of pleurolamprocystidia, together with the absence of pleuromacrocystidia, isolates it from the other sections within the subgenus.
Out of the five characteristics we focused on, three can be used, in combination with each other or other characteristics, to delimit subgenera or sections within the genus. Other morphological characteristics will need to be studied in more detail to morphologically support all subgenera and sections found in our phylogeny. Our study, together with previous ones (Verbeken 1996a, Verbeken & Walleyn 2010), indicates that microscopic characteristics such as the shape of pseudocystidia, the shape and ornamentation of the basidiospores (although difficult to quantify) or the shape of marginal cells might be important characteristics in certain groups. Other important characteristics that might be important in the evolution of Lactifluus species relate to their ecology, such as their ectomycorrhizal host trees. Within Lf. subg. Lactariopsis, the pileus development may also be an important morphological character: several species are characterised by involute pileus margins in young basidiomes, so that lamellae are protected when growing. On the contrary, in most other species pileus margins are not involute and lamellae are exposed from the beginning (De Crop et al. unpubl. res.). To know more about the evolutionary importance of this feature, a more detailed study on the ontogeny of basidiomes in the field is necessary.
Conclusions at species level
This study mainly focuses on the infrageneric relationships within Lactifluus and is not aimed at delimiting species within the genus. Our phylogeny cannot be used to make decisions at species level, although it can be used to draw attention to several species that need to be studied in more detail, using more collections and species delimitation techniques. The first clades within Lf. subg. Lactariopsis that draw our attention are those of Lf. madagascariensis and Lf. leoninus. For both species, the type specimen is on a longer branch than the other collection morphologically determined as the same species. This might be due to the poor quality of the type sequences. Further study is needed to verify if the latter is conspecific with the type specimens. In Lf. sect. Russulopsidei, Lf. ruvubuensis and Lf. longipes also need to be studied in more detail. The type of Lf. ruvubuensis is phylogenetically closest to a collection identified as Lf. longipes and not closest to the other collection identified as Lf. ruvubuensis. Even when adding more collections to the analysis, the Lf. ruvubuensis type clusters together with specimens determined as Lf. longipes. (unpubl. res.). This could indicate misdeterminations of the non-type collections, but a more thorough study is necessary to resolve this issue. Finally, there are several clades where multiple species cluster together. For example, within Lf. sect. Edules: Lf. aureifolius, Lf. indusiatus and Lf. fazaoensis, in Lf. sect. Pseudogymnocarpi: Lf. gymnocapoides, Lf. longisporus, Lf. pseudogymnocarpus and Lf. pumilus, in Lf. sect. Gymnocarpi: Lf. albocinctus and Lf. tanzanicus and in Lf. subg. Gymnocarpi, clade 9: Lf. cf. castaneibadius and Lf. cf. murinipes. Some of these species might have to be synonymised, or they may represent species complexes, the occurrence of which has repeatedly been reported in Lactifluus (Stubbe et al. 2010, Van de Putte et al. 2010, 2012, De Crop et al. 2012).
Morphological differences between the milkcap genera Lactifluus and Lactarius
It remains difficult to find morphological synapomorphies for either Lactarius or Lactifluus. Some general trends were formulated by Verbeken & Nuytinck (2013) that can be used to distinguish both genera:
thick-walled elements in the pileipellis and stipitipellis, as well as lamprocystidia, are generally present in Lactifluus and very rarely observed in Lactarius;
a hymenophoral trama composed of sphaerocytes (as in Russula) is common in Lactifluus but is rarely observed in Lactarius;
pleurotoid species are apparently restricted to Lactifluus;
sequestrate species are apparently restricted to Lactarius; and
species with velum are apparently restricted to Lactifluus.
Besides these morphological trends, the genera also differ in distribution. Lactarius is mainly distributed in the Northern hemisphere, while Lactifluus has its main range in the tropics. Despite these trends, both milkcap genera remain difficult to distinguish for the time being, and can only be separated with certainty through molecular data.
Ecology
Species of the genus Lactifluus can be found in temperate, subtropical and tropical regions, in a wide range of vegetation types, such as tropical and subtropical rain forests, subtropical dry forests, monsoon forests, tree savannahs, Mediterranean woodlands, temperate broadleaf and coniferous forests and montane forests. Basidiocarps are commonly found on soil, but sporadically on stems or aerial roots of trees, such as Lf. brunellus (Fig. 5e) on stems of Dicymbe corymbosa (Miller et al. 2002). Lactifluus species are ectomycorrhizal fungi and we hypothesize that the ectomycorrhizal hosts might have played important roles in species evolution. Present data suggest that both generalists and specialists occur, but the exact mycorrhizal connection generally remains undetermined. Ecological characteristics are not commonly recorded for every collection during field work, and it is hard to find out which tree a fungal species grows with in mixed forests. Common techniques to detect the host tree in mixed forests are labour-intensive and expensive, since ectomycorrhizal roots have to be excavated and both fungus and plant have to be sequenced.
Biogeography
As previously noted (Verbeken & Nuytinck 2013), Lactifluus is mainly distributed in the tropics. Tropical Africa is most species-rich, followed by tropical Asia and the Neotropical region. However, the Neotropics are still largely underexplored, so we expect the diversity of Lactifluus to be larger than currently known in the Neotropics. The geographical distribution of Lactifluus differs among the four subgenera. Lactifluus subg. Lactariopsis, Lf. subg. Gymnocarpi and Lf. subg. Pseudogymnocarpi mainly contain species from the tropics, but each contains one or two temperate lineages. Lactifluus subg. Lactifluus is mainly distributed in the northern hemisphere, with the exception of some Australian species, but with no known representatives in Africa or South America. Within Lactifluus, both allopatric and sympatric speciation are hypothesised to have played a role in the evolution of new species. Stubbe et al. (2010) noted that sympatric species of Lf. sect. Gerardii are often distantly related, which suggests allopatric speciation as the major mechanism responsible for the species diversity within this section. In contrast, Van de Putte et al. (2012) found that in Lf. subg. Lactifluus several closely related species occur in sympatry and therefore might have evolved reproductive barriers and/or different ways to exploit their environment. The biogeographical history of the genus will be discussed in more detail in our next publication, where we will use Bayesian techniques to date the Lactifluus phylogeny, to find out where the genus might have originated and how it reached its current distribution.
Acknowledgments
The first author is supported by the ‘Special Research Fund Ghent University’ (BOF, grant B/13485/01). The survey in Zambia was financially supported by the Research Foundation Flanders (FWO, grant K202014N) and by the Alberta Mennega Stichting. We would like to express our gratitude to all who helped during field work, especially to Deo Baribwegure, André-Ledoux Njounkouo and Donatha Tibuhwa. We would like to thank Viki Vandomme, Felix Hampe and Andy Vierstraete for conducting lab work. We thank Umberto Pera, Terry Henkel, Michael Kuo, Dieter Slos, Andy Methven, Gianluigi Bogi (www.bogiphoto.com), Ruben Walleyn† and Todd Elliot for providing pictures of Lactifluus species. We thank Shaun Pennycook and Scott Redhead for their help with the nomenclatural changes and Xiang-Hua Wang for her comments on the manuscript. We would like to thank everyone who provided material for this study: Adamčík S, Aime MC, Bâ A, Bandala VM, Basso MT, Bhandary HR, Buyck B, Carriconde F, Cifuentes J, Courtecuisse R, Das K, De Kesel A, Degreef J, Delivorias P, D’hooge E, Dibaluka Mpulusu S, Eyssartier G, Fiard JP, Gates G, Guo J, Hampe F, Henkel T, Horak E, Justice J, Lanquetin P, Le TH, Lecomte M, Lecuru C, Leonard P, Mata JL, Matheny PB, Montoya L, Morozova O, Noé F, Noordeloos M, Petersen R, Popov E, Rammeloo J, Ratkowsky D, Rock S, Saarimäki T, Sapnelis S, Sharma S, Singer R†, Sunar, Tonkin JE, Triantafyllou M, Vellinga EC, Walleyn R† and Wang XH. We thank the National Science Foundation (USA) for funding to Halling RE (DEB grants #0414665 and #1020421), the National Geographic Society for funding to Halling RE (CRE grant #8457-08), the Agence Nationale de la Recherche (CEBA, ref ANR-10-LABX-25-01) and the scientific station of Nouragues Reserve (CNRS, grant MYCOTIN) for funding to Roy M, and the Thailand Research Fund (TRF, grant BRG 5580009) for the financial support of the study of KD Hyde.
REFERENCES
- Binder M, Bresinsky A. 2002. Derivation of a polymorphic lineage of Gasteromycetes from boletoid ancestors. Mycologia 94, 1: 85–98. [PubMed] [Google Scholar]
- Bon M. 1983. Notes sur la systematique du genre Lactarius. Documents Mycologiques 13, 50: 15–26. [Google Scholar]
- Buyck B, Hofstetter V, Eberhardt U, et al. 2008. Walking the thin line between Russula and Lactarius: the dilemma of Russula subsect. Ochricompactae. Fungal Diversity 28: 15–40. [Google Scholar]
- Buyck B, Hofstetter V, Verbeken A, et al. 2010. Proposal 1919: To conserve Lactarius nom. cons. (Basidiomycota) with a conserved type. Mycotaxon 111: 504–508. [Google Scholar]
- Buyck B, Horak E. 1999. New taxa of pleurotoid Russulaceae. Mycologia 91, 3: 532–537. [Google Scholar]
- Buyck B, Verbeken A, Eberhardt U. 2007. The genus Lactarius in Madagascar. Mycological Research 111: 787–798. [DOI] [PubMed] [Google Scholar]
- Calonge FD, Martín MP. 2000. Morphological and molecular data on the taxonomy of Gymnomyces, Martellia and Zelleromyces (Russulales). Mycotaxon 76: 9–15. [Google Scholar]
- Cleland JB, Cheel EC. 1919. Australian fungi: notes and descriptions. Transactions and Proceedings of the Royal Society of South Australia 43: 262–315. [Google Scholar]
- De Crop E, Nuytinck J, Van de Putte K, et al. 2014. Lactifluus piperatus (Russulales, Basidiomycota) and allied species in Western Europe and a preliminary overview of the group worldwide. Mycological Progress 13, 3: 493–511. [Google Scholar]
- De Crop E, Tibuhwa D, Baribwegure D, et al. 2012. Lactifluus kigomaensis sp. nov. from Kigoma province,Tanzania. Cryptogamie Mycologie 33, 4: 421–426. [Google Scholar]
- Desjardin DE. 2003. A unique ballistosporic hypogeous sequestrate Lactarius from California. Mycologia 95: 148–155. [DOI] [PubMed] [Google Scholar]
- Donk MA. 1971. Progress in the study of the classification of the higher Basidiomycetes. In: Petersen RH. (ed), Evolution in the higher Basidiomycetes: 3–25. The University of Tennessee Press, Knoxville, USA. [Google Scholar]
- Drummond AJ, Bouckaert RR. 2015. Bayesian evolutionary analysis with BEAST. Cambridge University Press, Cambridge. [Google Scholar]
- Eberhardt U, Verbeken A. 2004. Sequestrate Lactarius species from tropical Africa: L. angiocarpus sp. nov. and L. dolichocaulis comb. nov. Mycological Research 108: 1042–1052. [DOI] [PubMed] [Google Scholar]
- Gardes M, Bruns TD. 1993. ITS primers with enhanced specificity for Basidiomycetes – application to the identification of mycorrhizae and rusts. Molecular Ecology 2, 2: 113–118. [DOI] [PubMed] [Google Scholar]
- Heilmann-Clausen J, Verbeken A, Vesterholt J. 1998. The genus Lactarius Vol. 2 – Fungi of Northern Europe. Svampetryk, Danish Mycological Society, Denmark. [Google Scholar]
- Heim R. 1937. Observations sur la flore mycologique malgache V. Les Lactario-Russulés à anneau: Ontogénie et Phylogénie (3). Revue de Mycologie 2: 109–117. [Google Scholar]
- Heim R. 1938. Les Lactario-russulés du domaine oriental de Madagascar: essai sur la classification et la phylogénie des Astérosporales, vol 1 Laboratoire de cryptogamie du Muséum national d’histoire naturelle, Paris. [Google Scholar]
- Henkel TW, Aime MC, Miller SL. 2000. Systematics of pleurotoid Russulaceae from Guyana and Japan, with notes on their ectomycorrhizal status. Mycologia 92, 6: 1119–1132. [Google Scholar]
- Hennings P. 1902. Fungi camerunenses novi III. Botanische Jahrbücher für Systematik, Pflanzengeschichte und Pflanzengeographie 30: 39–57. [Google Scholar]
- Hesler LR, Smith AH. 1979. North American species of Lactarius. University of Michigan Press, Ann Arbor. [Google Scholar]
- Jia FZ, Lo N, Ho SYW. 2014. The impact of modelling rate heterogeneity among sites on phylogenetic estimates of intraspecific evolutionary rates and timescales. PLoS One 9, 5; doi: 10.1371/journal.pone.0095722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Toh H. 2008. Recent developments in the MAFFT multiple sequence alignment program. Briefings in Bioinformatics 9, 4: 286–298. [DOI] [PubMed] [Google Scholar]
- Kreisel H. 1969. Grundzüge eines natürlichen Systems der Pilze. Verlag VEB Gustav Fischer, Jena. [Google Scholar]
- Lanfear R, Calcott B, Ho SYW, et al. 2012. PartitionFinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Molecular Biology and Evolution 29, 6: 1695–1701. [DOI] [PubMed] [Google Scholar]
- Larsson E, Larsson KH. 2003. Phylogenetic relationships of russuloid basidiomycetes with emphasis on aphyllophoralean taxa. Mycologia 95, 6: 1037–1065. [DOI] [PubMed] [Google Scholar]
- Le HT, Nuytinck J, Verbeken A, et al. 2007a. Lactarius in Northern Thailand: 1. Lactarius subgenus Piperites. Fungal Diversity 24: 173–224. [Google Scholar]
- Le HT, Verbeken A, Nuytinck J, et al. 2007b. Lactarius in Northern Thailand: 3. Lactarius subgenus Lactoriopsis. Mycotaxon 102: 281–291. [Google Scholar]
- Lebel T, Dunk CW, May TW. 2013. Rediscovery of Multifurca stenophylla (Berk.) T.Lebel, C.W.Dunk & T.W.May comb. nov (Russulaceae) from Australia. Mycological Progress 12, 3: 497–504. [Google Scholar]
- Lebel T, Tonkin JE. 2007. Australasian species of Macowanites are sequestrate species of Russula (Russulaceae, Basidiomycota). Australian Systematic Botany 20, 4: 355–381. [Google Scholar]
- Li GJ, Hyde KD, Zhao RL, et al. 2016. Fungal diversity notes 253–366: taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity 78, 1: 1–237. [Google Scholar]
- Liu YJJ, Whelen S, Benjamin DH. 1999. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerase II subunit. Molecular Biology and Evolution 16, 12: 1799–1808. [DOI] [PubMed] [Google Scholar]
- Maba DL, Guelly AK, Yorou NS, et al. 2014. Two new Lactifluus species (Basidiomycota, Russulales) from Fazao Malfakassa National Park (Togo, West Africa). Mycological Progress 13, 3: 513–524. [Google Scholar]
- Matheny PB. 2005. Improving phylogenetic inference of mushrooms with RPB1 and RPB2 nucleotide sequences (Inocybe; Agaricales). Molecular Phylogenetics and Evolution 35, 1: 1–20. [DOI] [PubMed] [Google Scholar]
- Matheny PB, Liu YJJ, Ammirati JF, et al. 2002. Using RPB1 sequences to improve phylogenetic inference among mushrooms (Inocybe, Agaricales). American Journal of Botany 89, 4: 688–698. [DOI] [PubMed] [Google Scholar]
- McNeill J, Turland NJ, Monro AM, et al. 2011. XVIII International Botanical Congress: Preliminary mail vote and report of Congress action on nomenclature proposals. Taxon 60, 5: 1507–1520. [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES science gateway for inference of large phylogenetic trees. Proceedings of the Gateway Computing Environments Workshop (GCE): 1–8. [Google Scholar]
- Miller SL, Aime MC, Henkel TW. 2002. Russulaceae of the Pakaraima Mountains of Guyan. I. New species of pleurotoid Lactarius. Mycologia 94, 3: 545–553. [PubMed] [Google Scholar]
- Miller SL, Aime MC, Henkel TW. 2012. Russulaceae of the Pakaraima Mountains of Guyana 2. New species of Russula and Lactifluus. Mycotaxon 121: 233–253. [Google Scholar]
- Miller SL, Larsson E, Larsson KH, et al. 2006. Perspectives in the new Russulales. Mycologia 98, 6: 960–970. [DOI] [PubMed] [Google Scholar]
- Miller SL, McClean TM, Walker JF, et al. 2001. A molecular phylogeny of the Russulales including agaricoid, gasteroid and pleurotoid taxa. Mycologia 93, 2: 344–354. [Google Scholar]
- Moncalvo JM, Lutzoni FM, Rehner SA, et al. 2000. Phylogenetic relationships of agaric fungi based on nuclear large subunit ribosomal DNA sequences. Systematic Biology 49, 2: 278–305. [DOI] [PubMed] [Google Scholar]
- Montoya L, Bandala VM, Mata M. 2007. Studies on Lactarius: Two new records from Costa Rica and additional information from Mexico. Mycotaxon 99: 279–290. [Google Scholar]
- Morozova OV, Popov ES, Kovalenko AE. 2013. Studies on mycobiota of Vietnam I. Two species of Lactifluus (Russulaceae) with pleurotoid basidiomata. Mikologiya I Fitopatologiya 47, 2: 92–102. [Google Scholar]
- Nagy LG, Kocsube S, Csanadi Z, et al. 2012. Re-mind the gap! Insertion – deletion data reveal neglected phylogenetic potential of the nuclear ribosomal internal transcribed spacer (ITS) of fungi. PLoS One 7, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuytinck J, Verbeken A. 2003. Lactarius sanguifluus versus Lactarius vinosus – molecular and morphological analyses. Mycological Progress 2, 3: 227–234. [Google Scholar]
- Nuytinck J, Verbeken A, Delarue S, et al. 2003. Systematics of European sequestrate lactarioid Russulaceae with spiny spore ornamentation. Belgian Journal of Botany 136, 2: 145–153. [Google Scholar]
- Nylander JAA, Wilgenbusch JC, Warren DL, et al. 2008. AWTY (are we there yet?): a system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bioinformatics 24, 4: 581–583. [DOI] [PubMed] [Google Scholar]
- Oberwinkler F. 1977. Das neue System der Basidiomyceten. In: Frey W, Hurka H, Oberwinkler F. (eds), Beiträge zur Biologie der niederen Pflanzen: 59–104. Stuttgart, New York: Gustav Fischer Verlag. [Google Scholar]
- Rambaut A, Suchard MA, Xie D, et al. 2014. Tracer v1.6. Available from http://beast.bio.ed.ac.uk/Tracer. [Google Scholar]
- Redhead SA, Norvell LL. 1993. Notes on Bondarzewia, Heterobasidion and Pleurogala. Mycotaxon 48: 371–380. [Google Scholar]
- Romagnesi H. 1948. Les problèmes et les méthodes de la systématique des champignons supérieurs. Bulletin de la Société Mycologique de France 64, 1-2: 53–100. [Google Scholar]
- Ronquist F, Teslenko M, Van der Mark P, et al. 2012. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61, 3: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sá MCA, Baseia IG, Wartchow F. 2013. Lactifluus dunensis, a new species from Rio Grande do Norte, Brazil. Mycosphere 4, 2: 261–265. [Google Scholar]
- Sá MCA, Wartchow F. 2013. Lactifluus aurantiorugosus (Russulaceae), a new species from Southern Brazil. Darwiniana, nueva serie 1, 1: 54–60. [Google Scholar]
- Singer R. 1942. Das System der Agaricales. II. Annales Mycologici 40: 1–132. [Google Scholar]
- Singer R. 1952. Russulaceae of Trinidad and Venezuela. Kew Bulletin 7: 295–301. [Google Scholar]
- Singer R. 1961. Diagnoses Fungorum novorum Agaricalium II. Sydowia Annales Mycologici 15, 1-6: 45–83. [Google Scholar]
- Singer R, Araujo I, Ivory MH. 1983. The ectotropically mycorrhizal fungi of the neotropical lowlands, especially Central Amazonia. (Litter decomposition and ectomycorrhiza in Amazonian forests 2.) Beihefte zur Nova Hedwigia 77: 1–352. [Google Scholar]
- Smith ME, Henkel TW, Aime MC, et al. 2011. Ectomycorrhizal fungal diversity and community structure on three co-occurring leguminous canopy tree species in a Neotropical rainforest. New Phytologist 192, 3: 699–712. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 9: 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J. 2008. A rapid bootstrap algorithm for the RAxML web servers. Systematic Biology 57, 5: 758–771. [DOI] [PubMed] [Google Scholar]
- Stiller JW, Hall BD. 1997. The origin of red algae: Implications for plasmid evolution. Proceedings of the National Academy of Sciences of the United States of America 94, 9: 4520–4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbe D, Le HT, Wang XH, et al. 2012a. The Australasian species of Lactarius subgenus Gerardii (Russulales). Fungal Diversity 52, 1: 141–167. [Google Scholar]
- Stubbe D, Nuytinck J, Verbeken A. 2010. Critical assessment of the Lactarius gerardii species complex (Russulales). Fungal Biology 114, 2-3: 271–283. [DOI] [PubMed] [Google Scholar]
- Stubbe D, Verbeken A, Wang X-H. 2012b. New combinations in Lactifluus. 2. L. subgenus Gerardii. Mycotaxon 119: 483–485. [Google Scholar]
- Tamura K, Stecher G, Peterson D, et al. 2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution 30, 12: 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedersoo L, May TW, Smith ME. 2010. Ectomycorrhizal lifestyle in fungi: global diversity, distribution, and evolution of phylogenetic lineages. Mycorrhiza 20, 4: 217–263. [DOI] [PubMed] [Google Scholar]
- Van de Putte K, De Kesel A, Nuytinck J, et al. 2009. A new Lactarius species from Togo with an isolated phylogenetic position. Cryptogamie Mycologie 30, 1: 39–44. [Google Scholar]
- Van de Putte K, Nuytinck J, Das K, et al. 2012. Exposing hidden diversity by concordant genealogies and morphology - a study of the Lactifluus volemus (Russulales) species complex in Sikkim Himalaya (India). Fungal Diversity 55, 1: 171–194. [Google Scholar]
- Van de Putte K, Nuytinck J, De Crop E, et al. 2016. Lactifluus volemus in Europe: three species in one – revealed by a multilocus genealogical approach, Bayesian species delimitation and morphology. Fungal Biology 120, 1: 1–25. [DOI] [PubMed] [Google Scholar]
- Van de Putte K, Nuytinck J, Stubbe D, et al. 2010. Lactarius volemus sensu lato (Russulales) from northern Thailand: morphological and phylogenetic species concepts explored. Fungal Diversity 45, 1: 99–130. [Google Scholar]
- Van Rooij P, De Kesel A, Verbeken A. 2003. Studies in tropical African Lactarius species (Russulales, Basidiomycota) 11. Records from Benin. Nova Hedwigia 77, 1-2: 221–251. [Google Scholar]
- Vellinga EC. 1988. Glossary. In: Noordeloos ME, Kuyper TW, Bas C. (eds), Flora Agaricina Neerlandica, Vol. 1: 54–64. Balkema, Rotterdam. [Google Scholar]
- Verbeken A. 1995. Further notes on Lactarius edulis Verbeken & Buyck. Russulales News 3: 18–23. [Google Scholar]
- Verbeken A. 1996a. Biodiversity of the genus Lactarius Pers. in tropical Africa. Part 1, text. Part 2, plates and maps. PhD thesis, Biology Department, Ghent University, Belgium. [Google Scholar]
- Verbeken A. 1996b. New taxa of Lactarius (Russulaceae) in tropical Africa. Bulletin du Jardin Botanique National de Belgique 65: 197–213. [Google Scholar]
- Verbeken A. 1998a. Studies in tropical African Lactarius species. 5. A synopsis of the subgenus Lactifluus (Burl.) Hesler & A.H. Sm. emend. Mycotaxon 66: 363–386. [Google Scholar]
- Verbeken A. 1998b. Studies in tropical African Lactarius species. 6. A synopsis of the subgenus Lactariopsis (Henn.) R. Heim emend. Mycotaxon 66: 387–418. [Google Scholar]
- Verbeken A. 2001. Studies in tropical African Lactarius species. 10. Infrageneric classification. Mycotaxon 77: 435–444. [Google Scholar]
- Verbeken A, Horak E. 1999. Lactarius (Basidiomycota) in Papua New Guinea. 1. Species of tropical lowland habitats. Australian Systematic Botany 12, 6: 767–779. [Google Scholar]
- Verbeken A, Nuytinck J. 2013. Not every milkcap is a Lactarius. Scripta Botanica Belgica 51: 162–168. [Google Scholar]
- Verbeken A, Nuytinck J, Buyck B. 2011. New combinations in Lactifluus. 1. L. subgenera Edules, Lactariopsis, and Russulopsis. Mycotaxon 118: 447–453. [Google Scholar]
- Verbeken A, Stubbe D, Van de Putte K, et al. 2014. Tales of the unexpected: angiocarpous representatives of the Russulaceae in tropical South East Asia. Persoonia 32: 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeken A, Van de Putte K, De Crop E. 2012. New combinations in Lactifluus. 3. L. subgenera Lactifluus and Piperati. Mycotaxon 120: 443–450. [Google Scholar]
- Verbeken A, Walleyn R. 1999. Studies in tropical African Lactarius species 7. A synopsis of the section Edules and a review on the edible species. Belgian Journal of Botany 132, 2: 175–184. [Google Scholar]
- Verbeken A, Walleyn R. 2010. Monograph of Lactarius in tropical Africa. Fungus Flora of Tropical Africa, vol. 2 National Botanic Garden, Belgium. [Google Scholar]
- Wang XH, Buyck B, Verbeken A. 2015. Revisiting the morphology and phylogeny of Lactifluus with three new lineages from southern China. Mycologia 107, 5: 941–958. [DOI] [PubMed] [Google Scholar]
- Wang XH, Stubbe D, Verbeken A. 2012. Lactifluus parvigerardii sp. nov., a new link towards the pleurotoid habit in Lactifluus subgen. Gerardii (Russulaceae, Russulales). Cryptogamie Mycologie 33, 2: 181–190. [Google Scholar]
- Wang XH, Verbeken A. 2006. Three new species of Lactarius subgenus Lactiflui (Russulaceae, Russulales) in southwestern China. Nova Hedwigia 83, 1-2: 167–176. [Google Scholar]
- White TJ, Bruns T, Lee S, et al. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, et al. (eds), PCR protocols: a guide to methods and applications: 315–322. Academic Press, New York. [Google Scholar]


