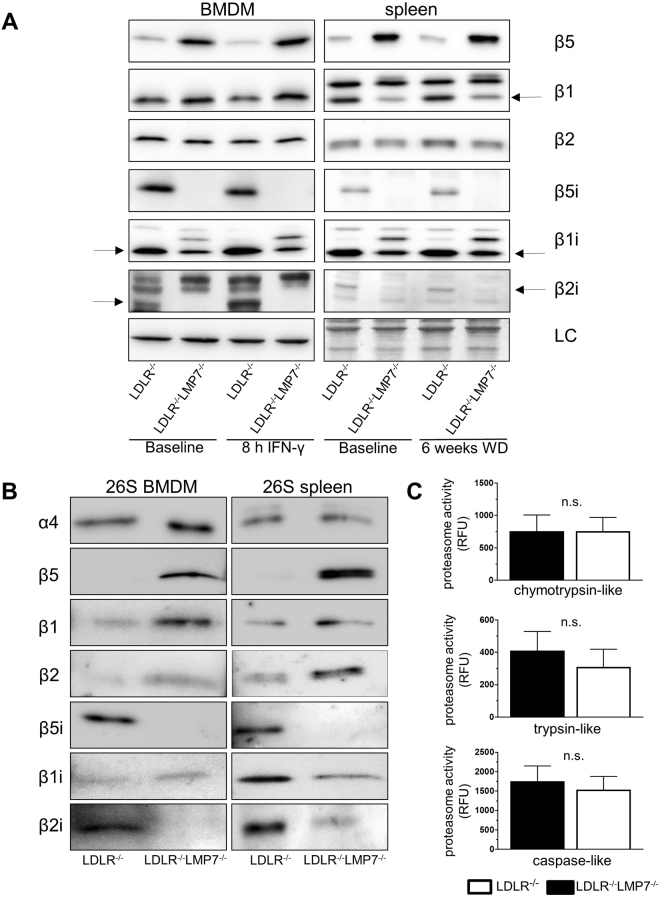
Figure 3.
Impact of β5i/LMP7-deficiency on proteasome composition and proteolytic activity in cells and tissues of LDLR−/− mice. (A) Western blot analysis of standard proteasome (β1, β2, and β5) and immunoproteasome (β1i, β2i, and β5i) subunit expression in BMDM of LDLR−/−LMP7−/− and LDLR−/− at baseline and after treatment with IFN-γ (100 U/ml) over 8 hours (left panel). Right panel shows Western blot analyses of pooled spleen protein samples from LDLR−/−LMP7−/− and LDLR−/− mice at baseline and after feeding a high-fat Western-type diet (WD) over 6 weeks (n = 11 mice per group). LC indicates actin staining (for BMDM) or amidoblack staining (for spleen) as loading controls. (B) Representative Western blots of standard proteasome and immunoproteasome subunits of isolated 26 S proteasome derived from murine LDLR−/−LMP7−/− and LDLR−/− BMDM (left panel) and spleen (right panel) lysates. (C) Chymotrypsin-, trypsin- and caspase-like proteasome activities (expressed as relative fluorescence units, RFU) of murine LDLR−/−LMP7−/− and LDLR−/− BMDM; n = 4 experiments. Unpaired t-test or Mann-Whitney U test. Data in graphs are presented as mean ± SEM.