Abstract
Background: The mammalian endoplasmic reticulum (ER) continuously adapts to the cellular secretory load by the activation of an unfolded protein response (UPR). This stress response results in expansion of the ER, upregulation of proteins involved in protein folding and degradation, and attenuation of protein synthesis. The response is orchestrated by three signalling pathways each activated by a specific signal transducer, either inositol requiring enzyme α (IRE1α), double-stranded RNA-activated protein kinase-like ER kinase (PERK) or activating transcription factor 6 (ATF6). Activation of IRE1α results in its oligomerisation, autophosphorylation and stimulation of its ribonuclease activity. The ribonuclease initiates the splicing of an intron from mRNA encoding the transcription factor, X-box binding protein 1 (XBP1), as well as degradation of specific mRNAs and microRNAs. Methods: To investigate the consequence of expression of exogenous XBP1, we generated a stable cell-line expressing spliced XBP1 mRNA under the control of an inducible promotor. Results: Following induction of expression, high levels of XBP1 protein were detected, which allowed upregulation of target genes in the absence of induction of the UPR. Remarkably under stress conditions, the expression of exogenous XBP1 repressed splicing of endogenous XBP1 mRNA without repressing the activation of PERK. Conclusions: These results illustrate that a feedback mechanism exists to attenuate activation of the Ire1α ribonuclease activity in the presence of XBP1.
Keywords: Unfolded protein response, IRE1α, XBP1, ER stress response.
Introduction
The endoplasmic reticulum (ER) is the site of protein folding and post-translational modification of secreted and transmembrane proteins 1. Under stress conditions such as glucose starvation or a viral infection, the folding capacity of the ER can become compromised, leading to a potentially lethal build-up of unfolded or misfolded proteins 2. Protein folding homeostasis can be restored by triggering of a stress response called the unfolded protein response (UPR) 3. This complex and tightly-regulated process has downstream effects that enable the ER to adapt to stress conditions, and if this pro-survival strategy does not successfully restore ER homeostasis then pro-apoptotic signalling is induced 4.
The mammalian UPR is formed from three distinct but overlapping signalling branches, each governed by an initial effector protein localised to the ER membrane. These proteins are activating transcription factor 6 (ATF6), protein kinase-like ER kinase (PERK) and inositol requiring enzyme α (IRE1α), and are activated in the presence of a build-up of incorrectly folded proteins 5. Of these three mammalian UPR effectors, IRE1α is the most conserved with its yeast homolog being solely responsible for the UPR in lower eukaryotes 6. The activation of its cytosolic endoribonuclease (RNase) domain enables processing of unspliced X-box binding protein 1 (XBP1) mRNA (XBP1u). Spliced transcripts (XBP1s) are translated into the protein XBP1 S, a transcription factor that upregulates the expression of proteins involved in ER protein folding, ER associated degradation (ERAD) and lipid biogenesis as part of a concerted effort to increase the capacity of the ER to cope with unfolded proteins.
In addition to its RNase domain, IRE1α also contains a cytosolic kinase domain and a lumenal domain that senses ER stress, and these domains are connected by a single transmembrane domain 7. Upon activation, IRE1α forms dimers 8, via ‘face-to-face’ interactions 9. The ‘face-to-face’ dimer displays no RNase activity and represents an early stage in IRE1α activation; its main purpose is to bring the kinase domains into proximity to enable transautophosphorylation. Phosphorylation induces a change in structure into a ‘back-to-back’ dimer 9, which brings the RNase domains into direct contact, forming a functional RNase active site capable of splicing XBP1u.
Activated IRE1α is also able to digest mRNAs 10 and miRNAs 11 during a process termed Regulated IRE1α Dependent Decay (RIDD). It has been suggested that the specificity of IRE1α changes during the UPR, initially cleaving XBP1u, but during prolonged stress switching to the cleavage of mRNA coding for proteins upregulated during the UPR. The consequence is an exacerbation of the stress leading to apoptosis 12. In addition, the cleavage of miRNAs responsible for the downregulation of caspase-2 results in elevated levels of this protease and induction of apoptosis through the BAX/BAK-dependent pathway 11. What regulates this switch in specificity is unknown, but could be related to subtle changes in IRE1α phosphorylation status, or conformation or interaction with IRE1α regulators 13.
Given the potential for IRE1α to activate proapoptotic factors during prolonged ER stress, it is important to understand how IRE1α activity is attenuated. Previous studies indicate that this attenuation may be the result of multiple mechanisms to reduce IRE1α protein, reverse oligomerisation or alter phosphorylation status. For example, IRE1α transcripts can be degraded by RIDD 14 and activated IRE1α dimers can be dephosphorylated by the phosphatase PP2Ce 15. In addition, the oxidation of thiols within the IRE1α lumenal domain occurs during activation, a modification that is reversed during IRE1α attenuation. This mechanism of attenuation is dependent upon oxidoreductase activity provided by P5, a member of the protein disulfide isomerase family 16. Finally the depletion of XBP1 S can be facilitated by XBP1 U, the protein translated from XBP1u transcripts, and involves the binding of XBP1 U to XBP1 S and subsequent trafficking to the 26S proteasome for degradation 17.
In order to explore the regulatory mechanisms of IRE1α, we investigated the impact of high levels of expression of XBP1 S on the activity of IRE1α. Our results show that an abundance of XBP1 S represses endogenous XBP1 splicing during unstressed and stress conditions. This repression may represent a regulatory mechanism, where persistent ER stress attenuates IRE1α RNase activity at least towards XBP1 mRNA.
Materials and methods
Generation of stable cell lines
CHO-S X was generated by transfecting 4 μg of pTetOne vector (Clontech), containing cDNA for the human XBP1s sequence, into CHO-S cells (Life Technologies), co-transfected with 200ng of a linear selectable marker for puromycin (a vector:marker ratio of 20:1), with 4.2 μl of the transfection reagent NovaCHOice ( Novagen). Transfected cells and untransfected control cells were grown in a 6 cm diameter dish in adherent culture, and after 24 h of growth were trypsinised and 1/10 of the cells were transferred to a 15 cm dish and grown in 20 ml medium containing 12.5 μg/ml puromycin. The transfected cells were grown for approximately 10 days, refreshing the selection medium every 3–4 days. Colonies were identified and removed from the dish using trypsin-soaked cloning discs and transferred into the wells of a 12 well plate, with one colony per well. The clones were grown under selection for another 3–5 days until the well was confluent, then the surviving clones were transferred into T25 flasks and later T75 flasks. To generate the CHO-S XB cell line, CHO-S X cells were transfected with a BFP construct 18 using the same method as described above. The construct contained a G418 resistance gene, and therefore the linear selectable marker was not required. Transfected cells were maintained under the dual selection of both 12.5 μg/mL puromycin and 2 mg/mL G418 (Promega) to maintain the BFP construct. Successful integration of the gene of interest was confirmed by western blotting.
Maintenance of cell lines
CHO-S and CHO-S XB cells were grown in DMEM supplemented with 10% foetal bovine serum (FBS), 2mM glutamine, and non-essential amino acids at a working concentration of 10 μM for each amino acid (Gibco). Cells were grown as an adherent culture and split every 3–4 days using a standard trypsin protocol.
XBP1 splicing assay
RNA was extracted from stress treated cells using Trizol Reagent (Ambion), following the manufacturer’s recommended protocol. First strand cDNA was synthesised using Superscript II Reverse Transcriptase (Invitrogen) with oligo dTs (Invitrogen), according to the manufacturer’s specifications. cDNA for endogenous XBP1 was amplified using primers designed using CLC Genomics Workbench (v6) (RRID:SCR_011853) to be specific to the Chinese hamster XBP1 sequence, (5’-CGCTTGGGAATGGATG-3’ and 5’- CAGGGTCCAACTTGTCC-3’; Sigma-Aldrich). The PCR reaction yielded a 247 bp fragment for XBP1u and a 215 bp fragment for XBP1s, plus a hybrid band of approximately 280 bp. Both endogenous and exogenous XBP1 were amplified simultaneously with a second, less specific set of primers, which can anneal to either the Chinese hamster or the human XBP1 sequence, 5’- ACAGCGCTTGGGGATGGATG-3’ and 5’- TGACTGGGTCCAAGTTGTCC-3’ (Sigma-Aldrich). PCR using these primers yielded the same fragments as the previous primer set, but with the addition of a fragment of 221 bp for exogenous XBP1s. PCR was performed with Accuzyme DNA polymerase (Bioline) with an initial melting step of 95°C for 5 min; then 35 cycles of 95°C for 45 s, an annealing step for 45 s, and 72°C for 45 s; followed by a final elongation step of 72°C for 10 min. The endogenous only primers used an annealing temperature of 60°C and the exogenous/endogenous primers used 62°C.
Cell lysis
After removing culture medium from the 6 cm diameter dish, the cells were washed with 20 mM NEM in PBS for 10 min. This was removed and 120 μl lysis buffer (50 mM Tris-HCl buffer containing 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 4mM NaF) was added to the monolayer and the cells were scraped into the buffer. This suspension was left on ice for 10 min, centrifuged at maximum speed for 10 min and the supernatant was extracted.
SDS PAGE
Crude lysates were mixed with 0.2 M Tris-HCl (pH 6.8) containing 10% (w/v) SDS, 20% (v/v) glycerol and 0.05% (w/v) bromophenol blue (sample buffer) in a 4:1 ratio of lysate to sample buffer. Dithiothreitol (DTT) was added as a reducing agent at a working concentration of 20 mM. Polyacrylamide gels were loaded with 20–30 μl of this sample mixture and run at 20 mA per gel.
Western blot
After separation, the samples were transferred to a nitrocellulose membrane (GE Healthcare) by wet transfer for 1 h at 250 mA using 25 mM Tris-HCl containing 200 mM glycine, 3.5 mM SDS and 20% (v/v) methanol. The blots were blocked in 5% (w/v) non-fat milk powder (Marvel) in 50 mM Tris-HCl buffer (pH 7.5) containing 150 mM NaCl and 0.1% (v/v) Tween (TBST) for 1 h. Primary antibodies were diluted in TBST and incubated overnight. Washes were performed three times for 10 min in TBST. Secondary antibodies were diluted in TBST and incubated for 1 h, and the blots shielded from light throughout the incubation. Blots were developed using the Odyssey SA scanner (Licor). The following primary antibodies were used: rabbit polyclonal anti-PDI 19, 1:500; rabbit polyclonal anti-XBP1 S (Biolegend, Cat# 619502, RRID:AB_315907), 1:500; rabbit monoclonal anti-PERK, (Cell Signalling, Cat# 3192 RRID:AB_2095847), 1:1000; mouse monoclonal anti-GAPDH, (Ambion, Cat# AM4300, RRID:AB_437392), 1:10000; rabbit polyclonal anti-actin, (Sigma-Aldrich Cat# A2103, RRID:AB_476694), 1:500. The following secondary antibodies were used: donkey polyclonal anti-mouse 680RD, (LI-COR Biosciences Cat# 926-68072, RRID:AB_10953628) 1:10000; donkey polyclonal anti-mouse 800CW (LI-COR Biosciences Cat# 926-32212, RRID:AB_621847), 1:10000; donkey polyclonal anti-rabbit 680RD, (LI-COR Biosciences Cat# 926-68073, RRID:AB_10954442), 1:10000; donkey polyclonal anti-rabbit 800CW (LI-COR Biosciences Cat# 926-32213, RRID:AB_621848), 1:10000.
ER Tracker treatment
ER Tracker Green BODIPY FL Glibenclamide (Molecular Probes) was dissolved in DMSO to a 1 mM stock concentration. Treated cells were stained with 250 nM ER Tracker in Hank’s Balanced Salt Solution (HBSS) for 30 min, then trypsinised and resuspended in DMEM.
ER stress treatments
Dithiothreitol (DTT) (Melford Labs) was prepared as 1 M stock in water and used at a 2.5 mM working concentration. Thapsigargin (Sigma-Aldrich) was prepared as a 2 mM stock in DMSO and used at 4 µM. Tunicamycin (Sigma-Aldrich) was prepared as a 10 mg/mL stock in DMSO and used at 10 µg/mL. PERK inhibitor (Tocris) was prepared as a 10mM stock in DMSO and used at 2.5 µM. Treatments were added for 3 h to cells that had been pretreated with doxycycline for 48 h.
Flow cytometry
Cells were washed once in HBSS then run on a FACS Canto II flow cytometer (BD Biosciences) in HBSS. ER Tracker Green has an excitation at 504 nm and emission at 511 nm, which can be detected using the FITC filter on the flow cytometer. The data obtained was analysed using Flowing Software v2.5.1 (Turku Bioimaging).
Results
To investigate the consequence of overexpression of human XBP1s in CHO-S cells, we created a stable cell-line expressing XBP1s mRNA under the control of a doxycycline inducible promoter. No XBP1 S was detected unless doxycycline was included in the medium ( Figure 1). Such tight regulation of expression allowed us to evaluate the effect of overexpression of XBP1s in the same cell line simply by culturing cells in the presence or absence of doxycycline.
Figure 1. Induction of XBP1S expression in CHO-S XB cells.
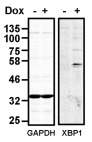
Western blot of lysates from CHO-S XB cells either uninduced or induced with doxycycline (Dox) for 3 days, probed with anti-XBP1s and anti-GAPDH as indicated. The blot is representative of the results obtained from three separate experiments.
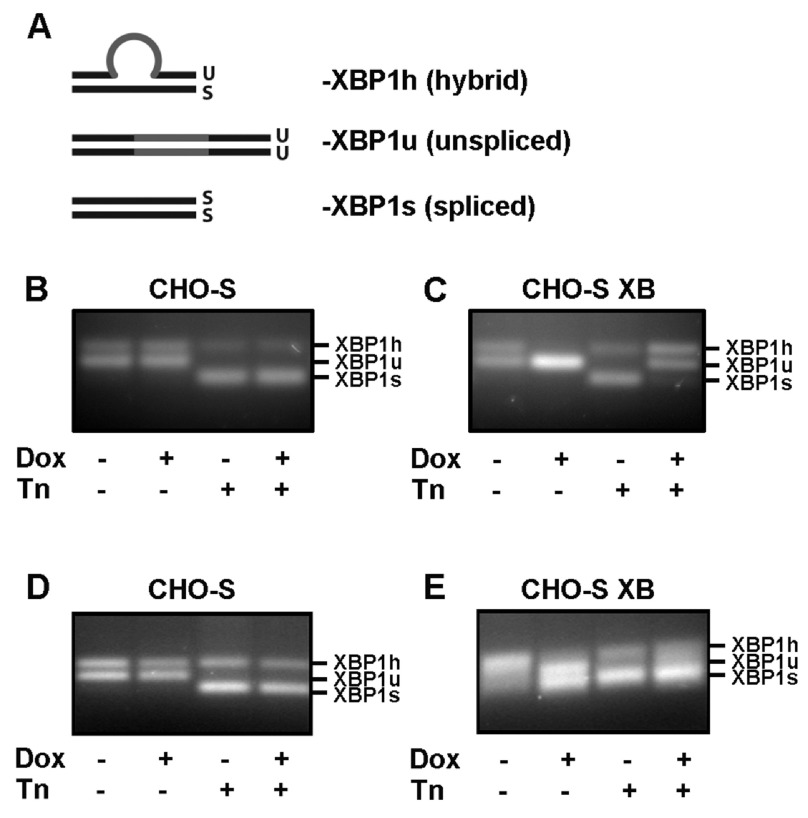
To study the activation of IRE1α RNase activity, we assessed the cleavage of XBP1u mRNA before and after induction of human XBP1s expression, using an RT-PCR assay 20. By designing primers that flank the XBP1 spliced intron, cDNA derived from XBP1s and XBP1u transcripts can be amplified by PCR and distinguished from each other by a subtle, 26bp difference in product size when run on an agarose gel. This assay is also known to generate a third PCR product, shown diagrammatically ( Figure 2A), which is thought to be a hybrid double-stranded cDNA product consisting of one strand XBP1s and one strand XBP1u. This hybrid product migrates above double-stranded XBP1u on an agarose gel due to its bulkier structure 20. Two sets of primers were designed for the assay: the first set was designed to only anneal to the endogenous hamster sequence of XBP1, and these primers were used to quantify only endogenous XBP1s and XBP1u. The second set was designed to bind to both forms of endogenous CHO XBP1, and also to the exogenous human XBP1s transcript, allowing visualisation of all forms of XBP1 present in the cell.
Figure 2. Consequence of XBP1s expression on XBP1u splicing in cell lines following endoplasmic reticulum stress.
( A) Schematic of the three possible cDNA products generated by primers that flank the XBP1 spliced intron (indicated in grey). Single stranded cDNA can anneal to either its complementary strand to generate double stranded XBP1u and XBP1s products, or generate a hybrid, XBP1h, formed from one strand XBP1u and one strand XBP1s. Of these three PCR products, XBP1s migrates the furthest on an agarose gel, followed by XBP1u and then by XBP1h, leading to the appearance of three distinct DNA bands. Adapted from 20. ( B– E) XBP1 splice assay of cDNA extracted from either ( B, D) CHO-S or ( C, E) CHO-S XB cells treated with doxycycline (Dox; for 3 days) and/or tunicamycin (Tn; for 3 h) as indicated. PCR reactions contained primers that anneal only to the endogenous CHO XBP1 sequence ( B, C) or primers that anneal to both the endogenous sequence and the exogenous XBP1s transcripts ( D, E). The experiment was performed at least three times, data from a representative experiment is shown.
In the absence of cell-stress, the endogenous XBP1 mRNA in CHO-S cells was present as a mixture of unspliced and hybrid forms ( Figures 2B and D). This result is consistent with a basal level of UPR signalling reported to be active under normal physiological conditions 21. As expected, there was no change in the splicing pattern following treatment with doxycycline, indicating that this chemical alone does not induce the UPR. Following treatment with the ER stress inducer tunicamycin, all of the XBP1 mRNA was converted to either the spliced or hybrid form indicative of a strong UPR ( Figures 2B and D). The splicing pattern of endogenous XBP1 mRNA in CHO-S XB cells in the absence of doxycycline, with or without cell stress was similar to that in CHO-S cells ( Figure 2C). However, in the absence of cell stress incubation with doxycycline to induce XBP1s expression prevented any splicing of endogenous XBP1 mRNA, as evidenced by the absence of the hybrid form. Doxycycline induced expression of exogenous XBP1s, as seen by the presence of XBP1s when primers recognising both the human and hamster XBP1 were used in the assay ( Figure 2E). We consistently observed an increase in expression of XBP1u following induction of XBP1s expression, indicating the upregulation of XBP1u expression by XBP1s, as shown previously 20, 22. Under conditions of ER stress XBP1 mRNA was efficiently spliced in the absence of doxycycline, but this splicing was dramatically repressed after doxycycline treatment ( Figure 2C). This result indicates that XBP1 splicing by IRE1α is largely prevented in cells overexpressing XBP1s.
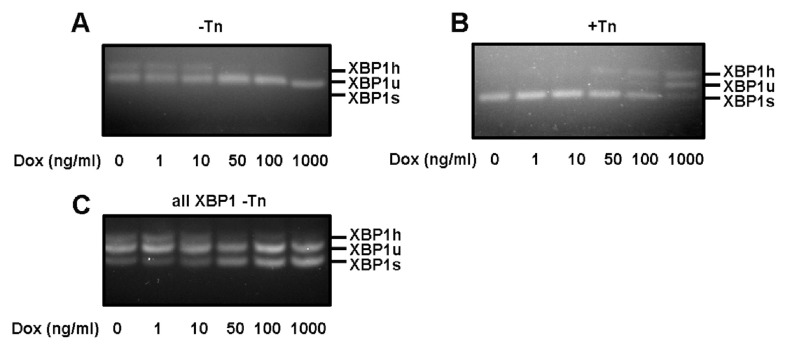
To determine whether there was a correlation between the induction of expression of exogenous XBP1s and the repression of IRE1α cleavage of endogenous XBP1 mRNA, we titrated the amount of added doxycycline to induce increasing amounts of exogenous XBP1s. The effect on cleavage of endogenous XBP1 mRNA became apparent after treating with 50 ng/ml of doxycycline both in the absence or presence of tunicamycin-induced ER stress ( Figures 3A and B). The effect increased with increasing concentrations of doxycycline with the greatest repression being most apparent at 1000 ng/ml in the presence of tunicamycin. When the presence of exogenously expressed XBP1s was evaluated using primers that amplify endogenous and exogenous XBP1, a clear increase in the XBP1s signal was observed at 50 ng/ml, which increased in intensity up to the highest concentration of doxycycline used ( Figure 3C). These results show a clear correlation between XBP1s expression and the repression of IRE1α-mediated cleavage of endogenous XBP1.
Figure 3. Inhibition of XBP1 splicing correlates with the amount of XBP1S.
XBP1 splice assay of cDNA from CHO-S XB cells induced for 3 days with a range of doxycycline (Dox) concentrations, either not treated with tunicamycin (Tn) ( A, C), or treated with 10µg/mL Tn for 3 h. Primers specific for endogenous XBP1 ( A, B) or endogenous and exogenous XBP1 ( C) were used. The experiment was performed twice, data from a representative experiment is shown.
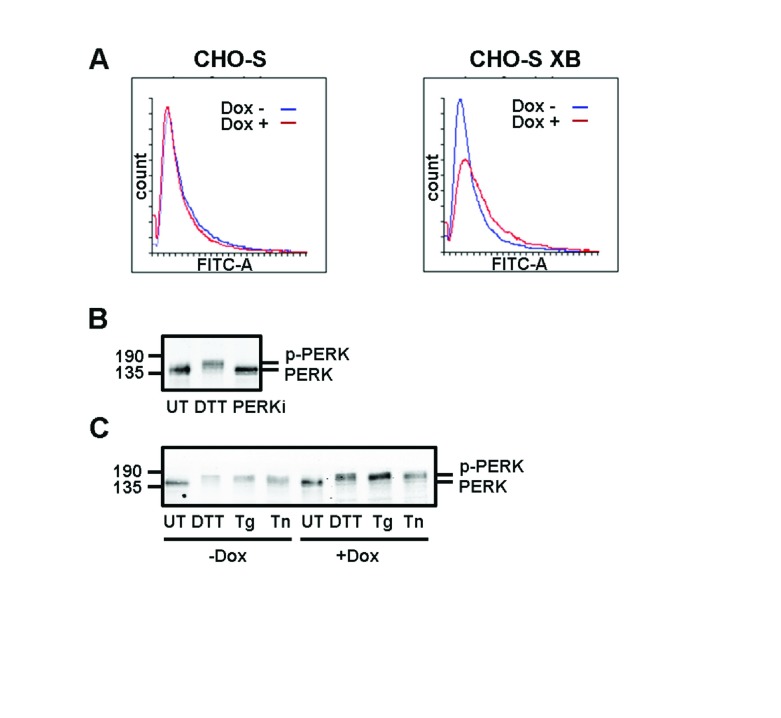
The expression of exogenous XBP1s should lead to the up-regulation of a number of proteins that are known to alleviate ER stress. Hence, the expression of XBP1s could prevent or suppress the tunicamycin-mediated activation of IRE1α, thereby repressing its RNase activity. To determine the consequence of XBP1s expression on ER expansion, we stained cells with ER Tracker, a dye that binds to potassium channels prominent at the ER membrane 23. Green fluorescence per cell was seen to increase following doxycycline treatment in CHO-S XB but not CHO-S cells, as quantified by FACS analysis ( Figure 4A). This result indicates that the expression of exogenous XBP1 does indeed cause an expansion in the ER, as seen previously when XBP1s is overexpressed in CHO cells 24. To determine whether XBP1s expression leads to a suppression of other branches of the UPR, we evaluated the activation of PERK, indicated by autophosphorylation. PERK phosphorylation was assayed by a shift in electrophoretic mobility to a slower migrating form after UPR induction, exemplified after treatment with DTT or the presence of a PERK kinase inhibitor 25 ( Figure 4B). There was no effect on PERK phosphorylation after treatment with a variety of UPR inducers (DTT, thapsigargin or tunicamycin) in the presence or absence of doxycycline in CHO-S XB cells. This result indicates that there is still a robust UPR activated following treatment with tunicamycin in cells overexpressing XBP1s as judged by PERK phosphorylation. This result suggests that the overexpression of XBP1s does not suppress the ability of UPR activators to induce a UPR.
Figure 4. PERK activation is unchanged by overexpression of XBP1S.
( A) Flow cytometry analysis of fluorescence from CHO-S and CHO-S XB cells stained with fluorescent ER Tracker dye. Samples were either treated with doxycycline (Dox) for 3 days (red) or left untreated (blue). ( B) Western blot of lysates from CHO-S XB cells that were either untreated (UT), treated with a reducing agent (DTT) or with an inhibitor of PERK kinase activity (PERKi). Blots were probed with anti-PERK to display the extent of PERK phosphorylation. ( C) Anti-PERK western blot of CHO-S XB cells induced with Dox and subsequently treated with DTT, thapsigargin (Tg) or tunicamycin (Tn). Each experiment was performed once.
Discussion
Activation of the IRE1α branch of the UPR can lead to a variety of outcomes based on regulation of its RNase activity 26. This activity is tightly controlled by a number of mechanisms, including the transcript and protein levels of IRE1α, changes to its quaternary structure and by its phosphorylation and redox state. Our results indicate an additional mechanism of IRE1α feedback regulation involving XBP1s, which is able to repress RNase activity towards XBP1u. This regulation was revealed upon overexpression of XBP1s and occurred in the absence and presence of ER stress.
Overexpression of XBP1s leads to high levels of expression of XBP1 S protein, to the expansion of the ER and an increased expression of secreted proteins 24, 27. Preconditioning the ER to stress by XBP1s expression could prevent IRE1α activation, thereby repressing splicing of endogenous XBP1u. However, we showed that robust activation of PERK still occurs in cells overexpressing XBP1s upon ER stress. As the mechanism for PERK and Ire1α activation requires BiP dissociation it seems unlikely that the absence of Ire1α activation is due to increased levels of BiP. Hence, activation of IRE1α should occur even in the presence of XBP1s. The repression of IRE1α ability to splice XBP1u is, therefore, most likely to occur downstream of its activation during stress conditions.
Activation of IRE1α leads to its phosphorylation, which has been shown to promote dimerisation of its cytosolic domain 28. This suggests that phosphorylation activates IRE1α, whereas a phosphatase could be responsible for attenuating IRE1α. One phosphatase, PP2Ce, has been suggested to perform this role 15; however, the promoter for this gene does not display the ERSE, ERSE-II or UPRE consensus sequences characteristic of genes upregulated by XBP1s 29, 30. Also, it has been shown that hyper-phosphorylation rather that dephosphorylation of yeast IRE1 is required to deactivate this protein 31. Nevertheless, the possibility remains that an XBP1s-inducible phosphatase could attenuate the activity of IRE1α during prolonged induction of the UPR.
Alternatively, XBP1 s could block the initial phosphorylation and dimerisation of IRE1α in order to reduce the overall intensity of IRE1α signalling. In support of this hypothesis, it was reported that XBP1 s works in complex with Sec63 and BiP to negatively regulate IRE1α autophosphorylation 32. A mouse Sec63 knockout cell line was shown to constitutively activate IRE1α phosphorylation, regardless of the presence of ER stress. Intriguingly, this study revealed that the overexpression of XBP1 s in the Sec63 knockout cell line was able to abolish the activation of IRE1α almost entirely, even in the presence of tunicamycin, indicating that Sec63 and XBP1 s work in concert to regulate IRE1α phosphorylation. However, this study did not examine the effect of XBP1 s overexpression on IRE1α activation in a cell line with physiological levels of Sec63; circumstances that would be closer to the conditions used in the results reported here.
The abundance of IRE1α can be modulated by proteosomal degradation initiated by ubiquitination by the E3 ubiquitin ligase synoviolin (SYVN1), otherwise known as the ERAD component HRD1 33. The ubiquitination of BiP-bound IRE1α monomers by SYVN1 leads to its dislocation from the ER and degradation by the proteasome. Under normal physiological conditions, BiP-bound IRE1α is continually degraded by ERAD, but the detachment of BiP allows for IRE1α to bypass interaction with SYVN1 and undergo accumulation and activation 34. Like other components of ERAD, SYVN1 is upregulated by XBP1s so it can be assumed that CHO-S XB would display high levels of SYNV1. This could lead to a reduction in IRE1α protein in XBP1s overexpressing cells; however, as only BiP-bound IRE1α is targeted for ERAD it is only this inactive form that would be affected by XBP1s upregulation, and not activated dimers. However, there is precedent for a reduction in total IRE1α unrelated to proteasomal degradation in the presence of stress. Heat shock treatment was shown to deplete IRE1α in a range of mammalian cell lines in a manner that could not be blocked by a proteasome inhibitor 35. The UPR was activated in these cells, indicated by the presence of IRE1α phosphorylation and other UPR hallmarks, but the specific mechanism for the degradation of IRE1α could not be clarified, and was attributed to an unknown method of suppressing extreme UPR signalling. It is possible that this mechanism could be mediated via XBP1s.
While we have noted here that XBP1s overexpression repressed XBP1u splicing activity, we have not investigated whether there is any suppression or even activation of activity towards other RNA substrates. It has been shown previously that overexpression of XBP1s had no effect on cell viability under non-stress conditions 12. Hence, under these conditions there was no suppression or activation of IRE1α RNase activity towards substrates other than XBP1u. It remains to be determined whether under stress conditions and in the presence of excess XBP1s, repression of IRE1α RNase activity extends to all mRNAs not just XBP1u.
Dysregulated IRE1α is a known contributing factor to a number of diseases, including multiple myeloma 36, epithelial cancers 37, Parkinson’s disease 38 and inflammatory bowel disease 39. Hence, increased knowledge of the regulatory mechanisms controlling IRE1α activity will help in understanding the pathogenesis of these diseases, as well as improving any therapeutic intervention.
Data availability
The data referenced by this article are under copyright with the following copyright statement: Copyright: © 2017 Chalmers F et al.
The uncropped western blots and the FACS files can be found on the Open Source Framework (DOI: 10.17605/OSF.IO/BGCDE; 40)
Acknowledgements
We thank Marie Anne Pringle for support during this work.
Funding Statement
The work was supported by the Wellcome Trust [103720]; and Biotechnology and Biological Sciences Research Council [BB/K004395/1].
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved with reservations]
References
- 1. Braakman I, Bulleid NJ: Protein folding and modification in the mammalian endoplasmic reticulum. Annu Rev Biochem. 2011;80:71–99. 10.1146/annurev-biochem-062209-093836 [DOI] [PubMed] [Google Scholar]
- 2. Kaufman RJ, Scheuner D, Schröder M, et al. : The unfolded protein response in nutrient sensing and differentiation. Nat Rev Mol Cell Biol. 2002;3(6):411–21. 10.1038/nrm829 [DOI] [PubMed] [Google Scholar]
- 3. Travers KJ, Patil CK, Wodicka L, et al. : Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell. 2000;101(3):249–58. 10.1016/S0092-8674(00)80835-1 [DOI] [PubMed] [Google Scholar]
- 4. Walter P, Ron D: The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–6. 10.1126/science.1209038 [DOI] [PubMed] [Google Scholar]
- 5. Kebache S, Cardin E, Nguyên DT, et al. : Nck-1 antagonizes the endoplasmic reticulum stress-induced inhibition of translation. J Biol Chem. 2004;279(10):9662–71. 10.1074/jbc.M310535200 [DOI] [PubMed] [Google Scholar]
- 6. Ron D, Walter P: Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519–29. 10.1038/nrm2199 [DOI] [PubMed] [Google Scholar]
- 7. Sidrauski C, Walter P: The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell. 1997;90(6):1031–9. 10.1016/S0092-8674(00)80369-4 [DOI] [PubMed] [Google Scholar]
- 8. van Anken E, Pincus D, Coyle S, et al. : Specificity in endoplasmic reticulum-stress signaling in yeast entails a step-wise engagement of HAC1 mRNA to clusters of the stress sensor Ire1. eLife. 2014;3:e05031. 10.7554/eLife.05031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Joshi A, Newbatt Y, McAndrew PC, et al. : Molecular mechanisms of human IRE1 activation through dimerization and ligand binding. Oncotarget. 2015;6(15):13019–35. 10.18632/oncotarget.3864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hollien J, Lin JH, Li H, et al. : Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol. 2009;186(3):323–31. 10.1083/jcb.200903014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Upton JP, Wang L, Han D, et al. : IRE1α cleaves select microRNAs during ER stress to derepress translation of proapoptotic Caspase-2. Science. 2012;338(6108):818–22. 10.1126/science.1226191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Han D, Lerner AG, Vande Walle L, et al. : IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell. 2009;138(3):562–75. 10.1016/j.cell.2009.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen Y, Brandizzi F: IRE1: ER stress sensor and cell fate executor. Trends Cell Biol. 2013;23(11):547–55. 10.1016/j.tcb.2013.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tirasophon W, Lee K, Callaghan B, et al. : The endoribonuclease activity of mammalian IRE1 autoregulates its mRNA and is required for the unfolded protein response. Genes Dev. 2000;14(21):2725–36. 10.1101/gad.839400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lu G, Ota A, Ren S, et al. : PPM1l encodes an inositol requiring-protein 1 (IRE1) specific phosphatase that regulates the functional outcome of the ER stress response. Mol Metab. 2013;2(4):405–16. 10.1016/j.molmet.2013.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eletto D, Eletto D, Dersh D, et al. : Protein disulfide isomerase A6 controls the decay of IRE1α signaling via disulfide-dependent association. Mol Cell. 2014;53(4):562–76. 10.1016/j.molcel.2014.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yoshida H, Oku M, Suzuki ML, et al. : pXBP1(U) encoded in XBP1 pre-mRNA negatively regulates unfolded protein response activator pXBP1(S) in mammalian ER stress response. J Cell Biol. 2006;172(4):565–75. 10.1083/jcb.200508145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Costantini LM, Baloban M, Markwardt ML, et al. : A palette of fluorescent proteins optimized for diverse cellular environments. Nat Commun. 2015;6:7670. 10.1038/ncomms8670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. John DC, Grant ME, Bulleid NJ: Cell-free synthesis and assembly of prolyl 4-hydroxylase: the role of the beta-subunit (PDI) in preventing misfolding and aggregation of the alpha-subunit. EMBO J. 1993;12(4):1587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shang J, Lehrman MA: Discordance of UPR signaling by ATF6 and Ire1p-XBP1 with levels of target transcripts. Biochem Biophys Res Commun. 2004;317(2):390–6. 10.1016/j.bbrc.2004.03.058 [DOI] [PubMed] [Google Scholar]
- 21. Wang Y, Xing P, Cui W, et al. : Acute Endoplasmic Reticulum Stress-Independent Unconventional Splicing of XBP1 mRNA in the Nucleus of Mammalian Cells. Int J Mol Sci. 2015;16(6):13302–21. 10.3390/ijms160613302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yoshida H, Okada T, Haze K, et al. : ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol Cell Biol. 2000;20(18):6755–67. 10.1128/MCB.20.18.6755-6767.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zunkler BJ, Wos-Maganga M, Panten U: Fluorescence microscopy studies with a fluorescent glibenclamide derivative, a high-affinity blocker of pancreatic beta-cell ATP-sensitive K + currents. Biochem Pharmacol. 2004;67(8):1437–44. 10.1016/j.bcp.2003.12.011 [DOI] [PubMed] [Google Scholar]
- 24. Cain K, Peters S, Hailu H, et al. : A CHO cell line engineered to express XBP1 and ERO1-Lα has increased levels of transient protein expression. Biotechnol Prog. 2013;29(3):697–706. 10.1002/btpr.1693 [DOI] [PubMed] [Google Scholar]
- 25. Axten JM, Medina JR, Feng Y, et al. : Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1 H-indol-5-yl)-7 H-pyrrolo[2,3- d]pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK). J Med Chem. 2012;55(16):7193–207. 10.1021/jm300713s [DOI] [PubMed] [Google Scholar]
- 26. Ghosh R, Wang L, Wang ES, et al. : Allosteric inhibition of the IRE1α RNase preserves cell viability and function during endoplasmic reticulum stress. Cell. 2014;158(3):534–48. 10.1016/j.cell.2014.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gulis G, Simi KC, de Toledo RR, et al. : Optimization of heterologous protein production in Chinese hamster ovary cells under overexpression of spliced form of human X-box binding protein. BMC Biotechnol. 2014;14:26. 10.1186/1472-6750-14-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee KP, Dey M, Neculai D, et al. : Structure of the dual enzyme ire1 reveals the basis for catalysis and regulation in nonconventional RNA splicing. Cell. 2008;132(1):89–100. 10.1016/j.cell.2007.10.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yamamoto K, Yoshida H, Kokame K, et al. : Differential contributions of ATF6 and XBP1 to the activation of endoplasmic reticulum stress-responsive cis-acting elements ERSE, UPRE and ERSE-II. J Biochem. 2004;136(3):343–50. 10.1093/jb/mvh122 [DOI] [PubMed] [Google Scholar]
- 30. Lee AH, Iwakoshi NN, Glimcher LH: XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23(21):7448–59. 10.1128/MCB.23.21.7448-7459.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rubio C, Pincus D, Korennykh A, et al. : Homeostatic adaptation to endoplasmic reticulum stress depends on Ire1 kinase activity. J Cell Biol. 2011;193(1):171–84. 10.1083/jcb.201007077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fedeles SV, So JS, Shrikhande A, et al. : Sec63 and Xbp1 regulate IRE1α activity and polycystic disease severity. J Clin Invest. 2015;125(5):1955–67. 10.1172/JCI78863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gao B, Lee SM, Chen A, et al. : Synoviolin promotes IRE1 ubiquitination and degradation in synovial fibroblasts from mice with collagen-induced arthritis. EMBO Rep. 2008;9(5):480–5. 10.1038/embor.2008.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sun S, Shi G, Sha H, et al. : IRE1α is an endogenous substrate of endoplasmic-reticulum-associated degradation. Nat Cell Biol. 2015;17(12):1546–55. 10.1038/ncb3266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Homma T, Fujii J: Heat stress promotes the down-regulation of IRE1α in cells: An atypical modulation of the UPR pathway. Exp Cell Res. 2016;349(1):128–138. 10.1016/j.yexcr.2016.10.006 [DOI] [PubMed] [Google Scholar]
- 36. Chen L, Li Q, She T, et al. : IRE1α-XBP1 signaling pathway, a potential therapeutic target in multiple myeloma. Leuk Res. 2016;49:7–12. 10.1016/j.leukres.2016.07.006 [DOI] [PubMed] [Google Scholar]
- 37. Jin C, Jin Z, Chen NZ, et al. : Activation of IRE1α-XBP1 pathway induces cell proliferation and invasion in colorectal carcinoma. Biochem Biophys Res Commun. 2016;470(1):75–81. 10.1016/j.bbrc.2015.12.119 [DOI] [PubMed] [Google Scholar]
- 38. Mercado G, Castillo V, Soto P, et al. : ER stress and Parkinson's disease: Pathological inputs that converge into the secretory pathway. Brain Res. 2016;1648(Pt B):626–632. 10.1016/j.brainres.2016.04.042 [DOI] [PubMed] [Google Scholar]
- 39. Kaser A, Martínez-Naves E, Blumberg RS: Endoplasmic reticulum stress: implications for inflammatory bowel disease pathogenesis. Curr Opin Gastroenterol. 2010;26(4):318–26. 10.1097/MOG.0b013e32833a9ff1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bulleid N: "Inhibition of IRE1α-Mediated XBP1 mRNA Cleavage by XBP1." Open Science Framework. 2017. Data Source [DOI] [PMC free article] [PubMed] [Google Scholar]