Table 1. Optimization of reaction conditions: the Rh-catalyzed cyclization of 1a with 2a a .
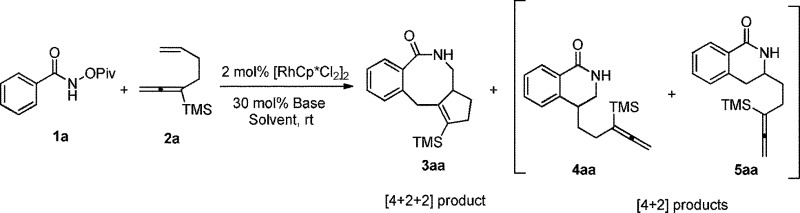
| |||||
| Entry | Solvent | Base | t/h | NMR yield
b
(%) |
|
| 3aa | 4aa + 5aa | ||||
| 1 | MeOH/H2O c | NaOAc | 11 | 75 | 12 |
| 2 | MeOH | NaOAc | 21 | 80 | 14 |
| 3 | DCE | NaOAc | 21 | 58 | 17 |
| 4 | DCM | NaOAc | 21 | 65 | 21 |
| 5 | Toluene | NaOAc | 21 | 44 | 17 |
| 6 d | MeOH | NaOAc | 14 | 80 | 16 |
| 7 e | MeOH | NaOAc | 14 | 74 | 15 |
| 8 | MeOH | KOAc | 14 | 68 | 14 |
| 9 | MeOH | CsOAc | 14 | 69 | 14 |
| 10 | MeOH | Na2CO3 | 14 | 69 | 14 |
| 11 | MeOH | K2CO3 | 14 | 81 (72) f | 14 |
| 12 | MeOH | Cs2CO3 | 14 | 76 | 14 |
| 13 | MeOH | K3PO4 | 11 | 73 | 13 |
| 14 g | MeOH | — | 11 | — | — |
| 15 h | MeOH | K2CO3 | 13 | 75 | 14 |
| 16 i | MeOH | K2CO3 | 13 | 72 | 13 |
| 17 j | MeOH | K2CO3 | 13 | 18 | 7 |
| 18 g , k | MeOH | K2CO3 | 13 | — | — |
aThe reaction was conducted with 1a (0.2 mmol), 2a (0.2 mmol), [Cp*RhCl2]2 (0.004 mmol), K2CO3 (0.06 mmol), MeOH (1.2 mL), and monitored by TLC.
bDetermined by 1H NMR using dibromomethane as internal standard.
cThe ratio of MeOH/H2O was 20/1 (1.2 mL/0.06 mL).
dUnder N2 atmosphere.
eUnder N2 atmosphere and 4 Å MS was added.
fIsolated yield in parentheses.
gRecovery of 1a was 98% with 2a disappeared.
h10 mol% K2CO3 was added.
i50 mol% K2CO3 was added.
j1 equiv. K2CO3 was added.
kThe reaction was conducted in the absence of the Rh(iii) catalyst.
