Fig. 7.
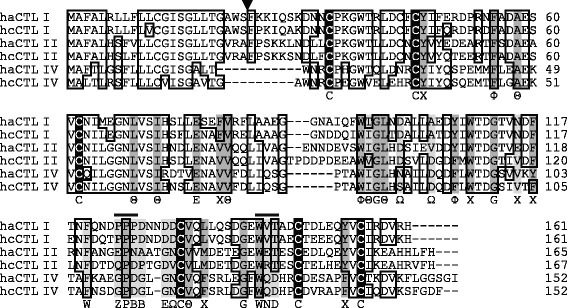
Amino-acid sequence alignment of C-type lectins (CTLs). Amino-acid sequence alignment was made from CTLs of Hippocampus abdominalis (i.e., haCTL I, II, and IV) together with those of H. comes (i.e., hcCTL I, II, and IV). Identical residues are boxed; dashes represent gaps; the triangle indicates the putative cleavage site of a signal sequence. Bars above sequences indicate the part of the Ca2+ binding site involved in sugar specificity. Conserved cysteine residues are shaded in black, and other conserved residues are shaded in dark gray; residues of the consensus motif that are not conserved in seahorse CTLs are shaded in light gray. Consensus residues of the lectins are shown below the sequences. Θ, aliphatic; Φ, aromatic; Χ, aliphatic or aromatic; Ω, side chain with carbonyl oxygen atom (D, N, E or Q); Z, E or Q; B, D or N [23]
