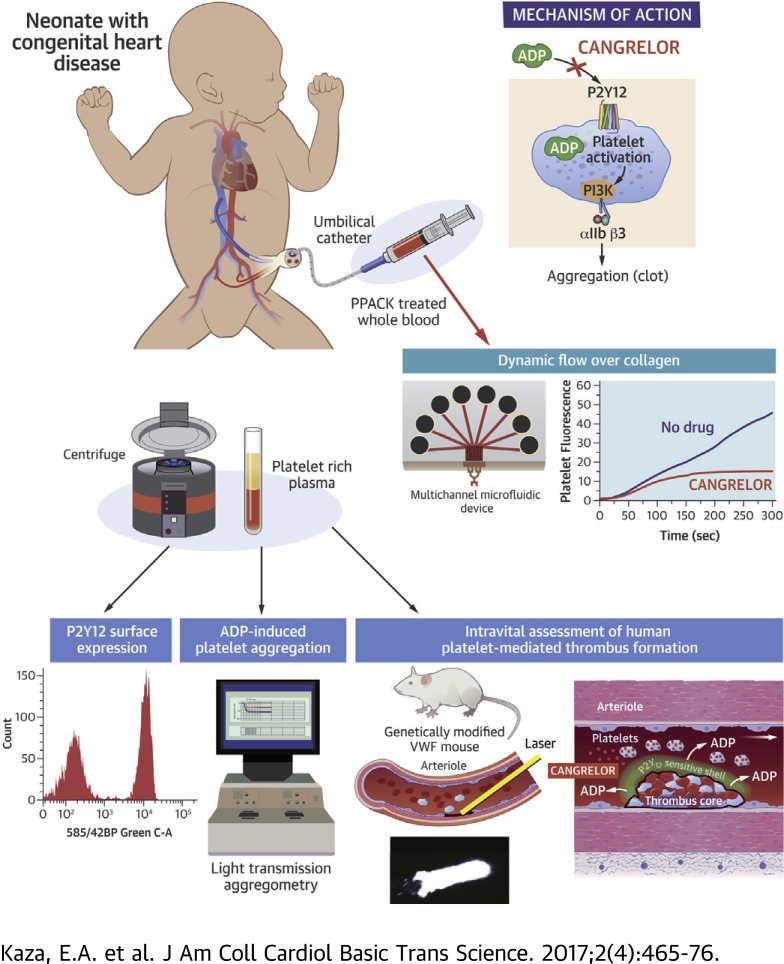
Visual Abstract
Key Words: antithrombotic, avatar mice, microfluidics, neonates, shunt thrombosis
Abbreviations and Acronyms: ADP, adenosine diphosphate; ATE, acute thromboembolic events; CHD, congenital heart disease; EC50, the concentration of a drug that gives half-maximal response; IC50, the concentration of an inhibitor where the response (or binding) is reduced by one-half; LTA, light transmission aggregometry; PCI, percutaneous coronary intervention; PD, pharmacodynamic; PK, pharmacokinetic; VWF, von Willebrand factor
Highlights
-
•
Platelets from neonatal patients with cyanotic congenital heart disease have a nearly identical response to adenosine diphosphate activation and P2Y12 receptor blockade with cangrelor as their adult counterparts.
-
•
Integrating high-throughput technologies with unique biological platforms can provide considerable insight into the potential clinical use of antiplatelet agents for neonatal and pediatric patients at risk for thrombosis.
-
•
Cangrelor may prove to be an effective antithrombotic drug with pharmacological properties well suited for use in the immediate post-operative period for neonates palliated with systemic-to-pulmonary artery shunts.
Summary
Shunt thrombosis remains a major cause of morbidity and mortality, especially during the initial palliation for single-ventricle physiology. The authors present evidence that the P2Y12 inhibitor cangrelor may fill a therapeutic void in thromboprophylaxis. They base this theory on results showing that platelets from neonatal patients with cyanotic congenital heart disease have a robust response to adenosine diphosphate and are amenable to P2Y12 inhibition with cangrelor. Unique to this study was their ability to establish drug efficacy in an avatar mouse model that permits the in vivo evaluation of human platelet–mediated thrombus formation illustrating that this P2Y12 inhibitor yields the intended biological response.
Acute thromboembolic events (ATEs) are rapidly becoming the new epidemic in centers that care for critically ill neonates due to an increase in invasive monitoring, life-saving technologies such as extracorporeal membrane oxygenation, and new surgical techniques and graft materials used to repair complex congenital heart disease (CHD) 1, 2. In the latter case, infants (aged <6 months) constitute the major proportion (approximately 70%) of patients seen in tertiary care centers with ATEs (3). In particular, those with single-ventricle physiology who require placement of a systemic-to-pulmonary artery shunt (e.g., modified Blalock-Taussig or central shunts) are at greatest risk, especially in the early post-operative period 3, 4, 5. Consequently, this scenario has resulted in suboptimal post-operative outcomes as exemplified in a retrospective review of 2,058 neonates who underwent palliation with a systemic-to-pulmonary artery shunt at multiple centers; discharge mortality and complication rates were around an aggregate of 6.7% and 12.3%, respectively (6). Early institution of aspirin, an irreversible inhibitor of platelet cyclooxygenase, is believed to be beneficial in reducing the risk of shunt occlusion and death 7, 8, but controversy remains regarding its overall effectiveness. In fact, it has been reported that aspirin alone may not be sufficient to fully inhibit platelet function in the immediate post-operative period (9). Thus, an urgent need remains for additional pharmacological protection to provide adequate thromboprophylaxis for these critically ill patients.
Clopidogrel (a thienopyridine derivative) is an antiplatelet agent that targets the adenosine diphosphate (ADP) receptor P2Y12 and is known to reduce the risk of ischemia and thrombosis in adult patients during and after percutaneous coronary intervention (PCI) 10, 11. It does so by impairing P2Y12 potentiation of platelet-dense granule secretion in response to strong agonists, stabilization of platelet aggregates by contributing to the activation of αIIbβ3, and inhibition of the antiplatelet effects of prostacyclin (12). Despite its proven clinical efficacy in adults with cardiovascular disease, clopidogrel has several major drawbacks that would limit its use in the immediate post-operative period for neonatal cardiac patients. These drawbacks include the requirement for oral administration that may result in erratic absorption (particularly in bypass cases), delay in the onset of action due to the need for conversion of the pro-drug to an active metabolite, and irreversible inhibition of the P2Y12 receptor that would necessitate platelet transfusion(s) if bleeding occurred (13). Interestingly, a previous clinical trial evaluating clopidogrel therapy in infants with cyanotic CHD who underwent palliation with a systemic-to-pulmonary artery shunt failed to show any benefit in reducing the rate of death or shunt-related morbidity in drug-treated patients (14). This outcome was somewhat surprising as the supraphysiologic shear rates (in excess of 15,000 s−1) and shearing forces predicted to occur in artificial conduits connecting the systemic circulation to the pulmonary artery would favor P2Y12-mediated platelet activation and aggregation (15). However, potential shortcomings of this trial were the low dosage of drug administered and the concomitant use of aspirin in the majority of patients, making it difficult to ascertain the contribution of the P2Y12-signaling pathway in supporting platelet aggregation and thrombus formation in these young patients (16).
Cangrelor, an adenosine triphosphate analogue, is a P2Y12 receptor antagonist given intravenously with properties more suitable for short-term use; these properties include a rapid, direct, predictable, and reversible inhibition of platelet function 17, 18. In particular, cangrelor given as a 30-μg/kg bolus followed by a 4-μg/kg/min infusion for 60 min to healthy adult volunteers resulted in a maximum concentration of 635 ng/ml, a drug half-life of 3.7 ± 1.1 min, and complete inhibition of ADP (5 μM)-induced platelet aggregation. However, there was restoration of hemostasis, as assessed by bleeding time, seen within 10 min of cessation of infusion and full platelet function recovery within 60 min; it is also rapidly inactivated in the circulation by dephosphorylation to the nucleoside, which is independent of liver or renal function. On the basis of these characteristics, cangrelor was selected for clinical development as an intravenous antithrombotic agent and approved by the U.S. Food and Drug Administration in 2015 for use in adults undergoing PCI 19, 20. These favorable pharmacokinetic (PK) and pharmacodynamic (PD) properties would make it an ideal drug for use in the immediate post-operative period in neonates with CHD requiring palliation with a systemic-to-pulmonary artery shunt, especially in patients who develop renal, hepatic, and gastrointestinal dysfunction associated with controlled surgical hypothermia and/or cardiopulmonary bypass (21). Moreover, the ability to achieve an antiplatelet effect within 10 to 15 min of starting a continuous infusion of drug without a previous bolus is of value in this post-operative population in whom a balance must be struck between providing adequate anticoagulation and maintaining sufficient hemostasis. Of note, simulations based on population PK/PD modeling (data on file, Chiesi USA, Inc., 2013) suggest that expected steady-state concentrations of cangrelor may be lower in young children, infants, and neonates than in adults.
The present preclinical study was designed to assess the contribution of the P2Y12-signaling pathway in supporting the ability of platelets from neonatal patients with CHD to promote thrombus growth and to determine the potential in vivo efficacy of cangrelor, information essential for proceeding to a Phase I PK/PD clinical trial.
Methods
Additional details regarding the Methods are presented in the Supplemental Appendix.
Patient population
Patients were eligible for enrollment in the study if they were between 0 and 18 years of age and with known CHD. Exclusion criteria included coagulation defects, known congenital or genetic conditions expected to affect platelet function, body weight <3 kg for neonatal patients and <6 kg for older pediatric patients, cardiopulmonary instability necessitating urgent or emergent surgical/catheter-based intervention, and medications (e.g., aspirin) or other conditions that affect platelet function.
Blood collection
For studies involving neonates and pediatric patients with CHD, a blood sample was obtained from a central venous catheter after clearing the line of heparin; blood samples from healthy adult volunteers were obtained via routine venipuncture. In the majority of cases, 3.8% trisodium citrate served as an anticoagulant. For microfluidic studies, whole blood (1 ml) was collected in a syringe containing the thrombin inhibitor H-D-Phe-Pro-Arg-chloromethylketone (PPACK) to maintain extracellular cation concentrations and thus preserve integrin function. For light transmission aggregometry (LTA) studies, citrated blood (9 ml) samples were centrifuged to generate platelet-rich plasma and ultimately purified platelets, which were suspended to 400,000/μl in buffer containing 145 mM of NaCl, 10 mM of Hepes, 0.5 mM of Na2HPO4, 5 mM of KCl, 2 mM of MgCl2, 0.1% glucose, 0.3% human serum albumin, and pH 7.4. Informed consent was obtained before all blood draws from healthy adults and parents of neonates and children by using a protocol approved by the institutional review committee at Columbia University Medical Center.
In vivo thrombus formation
Administration of anesthesia, insertion of vascular catheters, fluorescent labeling of platelets, and surgical preparation of the cremaster muscle of male mice and the generation of von Willebrand factor (VWF)HA1-mutant animals have been described previously 22, 23. A pulsed nitrogen dye laser was used to induce arteriole injury in the cremaster muscle of anesthetized 8- to 12-week-old animals. Human platelet–vessel wall interactions were visualized by using fluorescence microscopy. Experiments were performed in accordance with the guidelines set forth by the Institutional Animal Care and Use Committee at Columbia University Medical Center.
Platelet aggregation and adhesion in flow
The ability of ADP and cangrelor to promote or inhibit aggregation of human platelets, respectively, was assessed by using a Lumi-Aggregometer (model 540 VS, Chrono-Log Corp., Havertown, Pennsylvania). A parallel-plate flow chamber was used to assess human platelet accumulation on surface-immobilized plasma VWF 22, 23, whereas microfluidic devices were used to evaluate the thrombus growth on a patterned collagen surface (24).
Results
Patient population
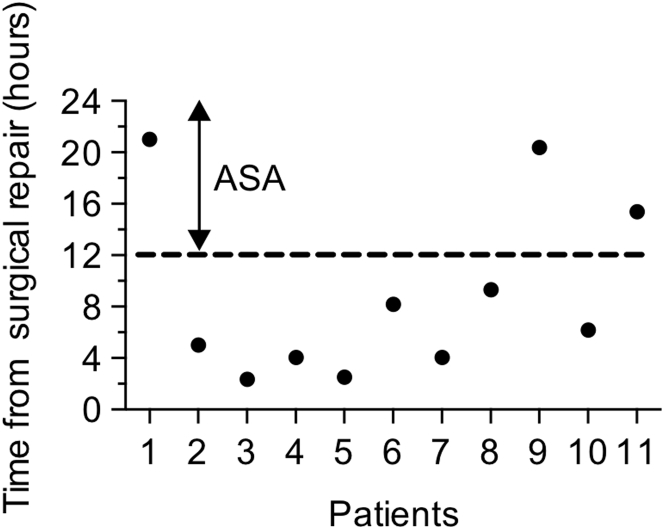
Eighty-eight pediatric patients with CHD, consisting of 3 distinct populations (Table 1), were enrolled in this study from September 2013 to October 2016. The first group included 49 full-term neonates (mean age 11.7 days) with a body weight ≥3 kg who were scheduled for cardiac surgery involving cardiopulmonary bypass. Patients with single-ventricle physiology accounted for 30.6% of the population enrolled in the study, with the majority having dexo-transposition of the great arteries (38.8%). During this time period, 11 neonates requiring palliation with a systemic-to-pulmonary shunt (including both enrolled and nonenrolled patients) had evidence of shunt occlusion necessitating a surgical intervention and/or rapid response extracorporeal membrane oxygenation (Supplemental Table 1). Interestingly, all events occurred within 24 h of surgery, with the majority of cases occurring before initiation of aspirin therapy (Figure 1). The second and third groups included pediatric patients ranging in age from 1 to 10 years (mean age 4.1 years) and 11 to 19 years (mean age 15.3 years), respectively, scheduled to undergo left heart catheterization for diagnostic or therapeutic purposes. Of note, none of the patients were receiving an antiplatelet medication (e.g., aspirin) at the time of study.
Table 1.
Baseline Demographic Characteristics of Cardiac Patients Enrolled in the Study
| Age 0–28 Days (n = 49) | Age 1–10 Years (n = 31) | Age 11–18 Years (n = 8) | |
|---|---|---|---|
| Age, yrs | 11.7 ± 3.1 | 4.1 ± 1.8 | 15.3 ± 4.7 |
| Female | 24.5 | 54.8 | 37.5 |
| Ethnicity | |||
| Hispanic/Latino | 20.4 | 25.8 | 12.5 |
| Race | |||
| White | 61.2 | 64.5 | 62.5 |
| Black | 6.1 | 6.5 | 12.5 |
| Asian | 12.2 | 3.2 | 12.5 |
| Native American | 0 | 0 | 0 |
| Other | 20.4 | 25.8 | 12.5 |
| Mean gestational age, weeks | 37.4 | ||
| Conditions | |||
| Hypoplastic left heart syndrome | 30.6 | 19.4 | 0 |
| Pulmonary atresia with intact ventricular septum | 0 | 3.2 | 0 |
| Tricuspid atresia | 0 | 6.5 | 0 |
| Transposition of great arteries | 38.8 | 12.9 | 0 |
| Tetralogy of Fallot | 2.0 | 19.4 | 25.0 |
| Double-inlet left ventricle | 4.1 | 3.2 | 0 |
| Double-outlet right ventricle | 0 | 3.2 | 12.5 |
| Coarctation | 8.2 | 3.2 | 12.5 |
| PDA | 0 | 12.9 | 0 |
| ASD | 0 | 0 | 25 |
| Heterotaxy | 4.1 | 0 | 0 |
| Other single-ventricle physiology | 0 | 0 | 0 |
| Other double-ventricle physiology | 12.2 | 16.1 | 25.0 |
Values are mean ± SD or %.
ASD = atrial septal defect; PDA = patent ductus arteriosus.
Figure 1.
Time From Surgery to Shunt Occlusion in Neonatal Cardiac Patients
The data represent the post-operative in-hospital population at Columbia University Medical Center from 2013 to 2016 who developed evidence of shunt occlusion commencing from their return from surgery. The dotted line denotes the time aspirin (ASA) is typically administered (12 to 24 h post-surgical repair).
P2Y12-mediated platelet adhesion and thrombus growth
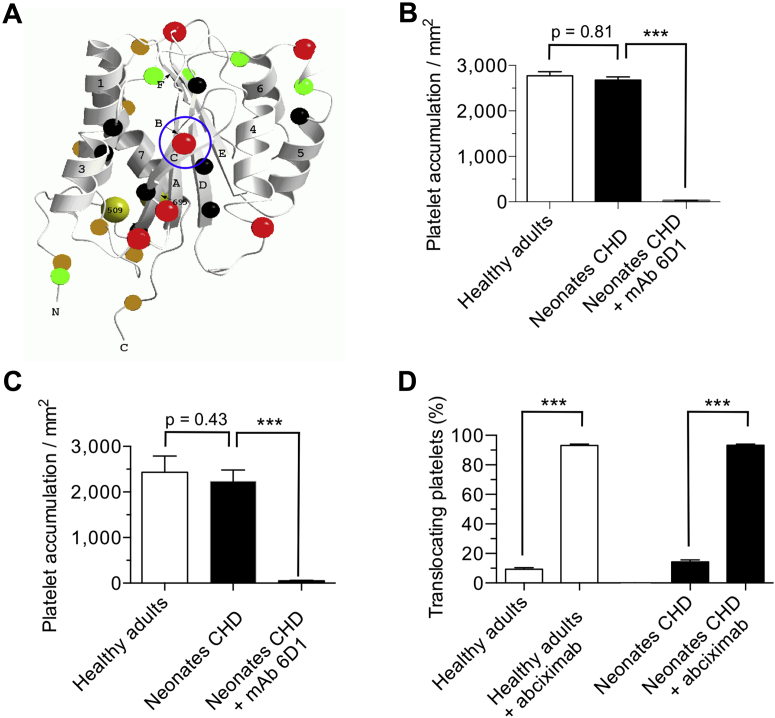
To assess the thrombogenic properties of platelets isolated from the 3 populations of patients under biologically relevant conditions, a genetically modified mouse model was used in which the human VWF A1 domain (VWFHA1) replaced its murine counterpart (Figure 2A); consequently, human but not mouse platelets are able to support hemostasis and thrombosis in response to vascular injury (23). The interaction between the human platelet receptor glycoprotein Ib alpha (GPIbα) and the A1 domain of VWF (VWF-A1) is key for initiating thrombus formation in humans and in this avatar mouse model; thus, we first compared the ability of surface-immobilized human plasma VWF versus murine plasma VWFHA1 to support platelet accumulation under arterial flow conditions (wall shear rate of 1,600 s−1). No significant difference was observed in the number of platelets from neonatal patients or healthy adults that attached to either human plasma VWF (2,675 ± 174 platelets/mm2 vs. 2,772 ± 188 platelets/mm2, respectively; p = 0.81) or murine plasma VWFHA1 (2,432 ± 112 platelets/mm2 vs. 2,577 ± 250 platelets/mm2; p = 0.43) (Figures 2B and 2C). The reliance on GPIbα–VWF interactions for supporting platelet accrual is demonstrated by the ability of the GPIbα function blocking monoclonal antibody 6D1 to nearly abrogate platelet adhesion in flow.
Figure 2.
Human Platelet Accrual on Plasma VWFHA1 in Flow
(A) Main chain schematic of the human von Willebrand factor (VWF)-A1 domain depicting residues that differ from its murine counterpart. Colored spheres denote the following: black are residues with buried side chains, green have partially buried side chains, red have exposed side chains on the front and upper surfaces, and gold have exposed side chains on the lower and back surfaces. Circled in blue is residue 1326, which plays a key role in permitting interactions with the GPIbα on human and mouse platelets (29). Accumulation of platelets on (B) surface-immobilized human plasma VWF or (C) murine plasma VWFHA1 is shown. Citrated whole blood from neonatal patients or healthy adults was perfused over the reactive substrate for 3 min (wall shear rate of 1,600 s−1) before assessing the number of interacting platelets. (D) Percentage of translocating platelets that became firmly adherent in response to infusion of buffer containing adenosine diphosphate (20 μM). The human GPIbα function blocking monoclonal antibody (mAb) 6D1 (10 μg/ml) or the αIIbβ3 inhibitor abciximab (10 μg/ml) was added to blood 10 min before use. See Supplemental Video 1. Data represent the mean ± SEM (n = 10 individuals per age group). ***p < 0.001 for antibody treatment versus no treatment according to Mann-Whitney U test. CHD = congenital heart disease.
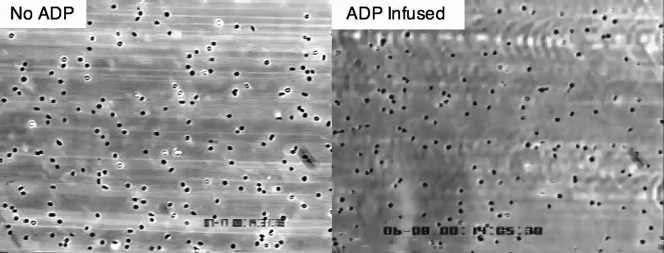
P2Y Signaling Axis Is Robust in Neonatal Platelets and Can Support Integrin-Dependent Firm Adhesion to Surface-Adherent VWF in Response to ADP
Citrated whole blood from a neonatal cardiac patient was infused over surface-immobilized plasma von Willebrand factor (VWF)HA1 for 3 min (wall shear rate of 1,600 s−1), followed by the addition of platelet buffer with or without adenosine diphosphate (ADP) (20 mmol/L) for 1 min. ADP is seen to trigger firm adhesion, which prevents the further forward motion of translocating platelets.
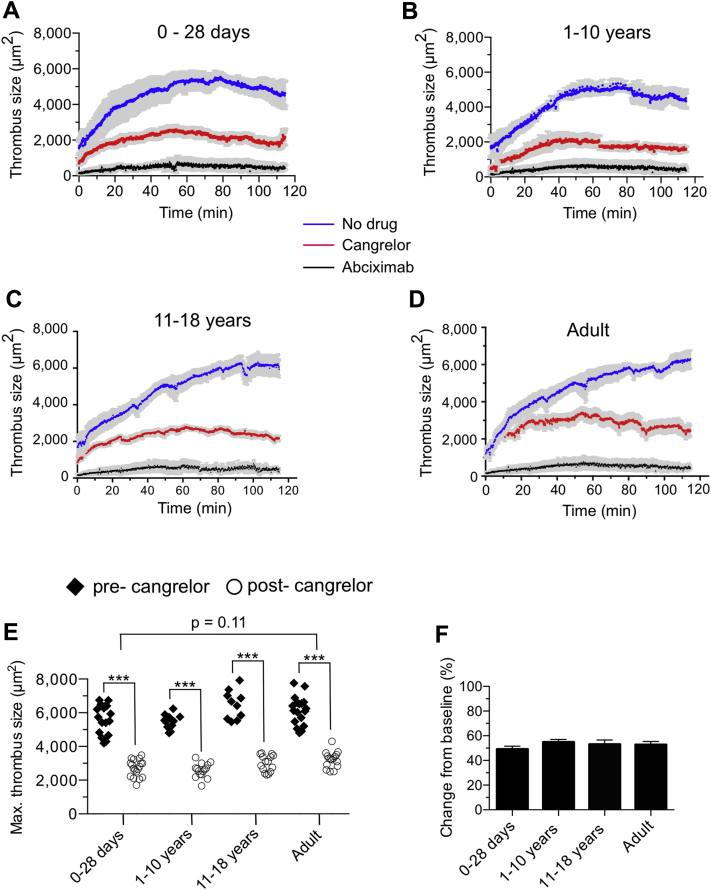
To determine whether the P2Y12-signaling pathway is robust in platelets from neonatal patients with CHD, we next evaluated the ability of ADP to enable platelets translocating on surface-immobilized VWF, in response to forces generated by flowing blood, to activate and firmly adhere to the substrate by engaging the integrin αIIbβ3 (25). GPIbα–VWF interactions are transient in nature due to the rapid kinetic properties of the bond formed between this receptor-ligand pair necessitating integrin-mediated firm adhesion for thrombus growth (26). Results indicate that this signaling axis is intact as infusion of buffer containing ADP stabilized interactions between platelets and VWF (Figure 2D, Supplemental Video 1), a process that could be prevented by pretreatment of citrated whole blood with the αIIbβ3 inhibitor abciximab. Definitive evidence was obtained by analyzing the in vivo behavior of fluorescently labeled human platelets in laser-injured arterioles of VWFHA1 mice before and after administration of cangrelor. The dose of drug administered to the mice was based on previous clinical trials in adults undergoing PCI (e.g., 30-μg/kg bolus followed by a 4-μg/kg/min infusion), which resulted in sustained blood levels >400 ng/ml in human volunteers 17, 27. Interestingly, maximal thrombus size generated by platelets isolated from neonatal cardiac patients was similar to adults (5,676 ± 203 μm2 vs. 6,141 ± 199 μm2, respectively; p = 0.11) (Figures 3A to 3E). Consistent with the requirement for ADP to promote thrombus growth (28) was the ability of cangrelor to reduce clot size by >45% in all age groups (Figures 3E and 3F, Supplemental Video 2). In contrast, administration of the αIIbβ3 inhibitor abciximab reduced thrombus formation by >90% (p < 0.001) (Figures 3A to 3D).
Figure 3.
Cangrelor Reduces Human Platelet Thrombus Formation in Avatar Mice
(A to D) Time course of fluorescent platelets accumulating on laser-injured arterioles contained with the cremaster muscle of von Willebrand factorHA1 mice. Cangrelor (30-μg/kg bolus; 4-μg/kg/min infusion) or abciximab (0.25-μg/kg bolus; 0.125-μg/kg/min infusion) were administered intravenously after establishing baseline thrombus formation in the absence of drug. n = 15 (age 0 to 28 days); n = 10 (age 1 to 10 years); n = 8 (age 11 to 18 years); n = 15 for adults. (E) Maximal thrombus size pre- and post-administration of cangrelor in the same animal. Each symbol represents the area of a thrombus in 1 arteriole of an avatar mouse. (F) Percent change in thrombus size in response to cangrelor treatment based on data shown in E. See Supplemental Video 2. Data represent mean ± SEM. ***p < 0.001 for drug versus no treatment according to the Mann-Whitney U test.
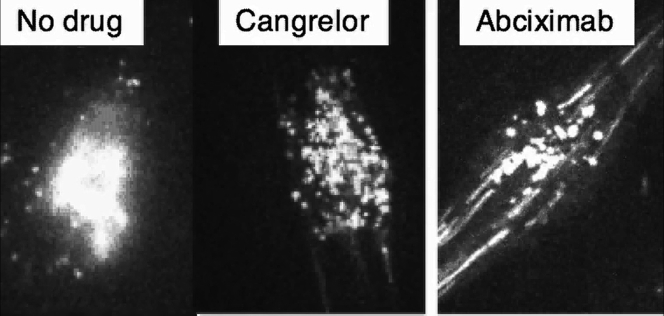
Cangrelor Reduces Human Platelet Thrombus Formation in Laser-Injured Arterioles of VWFHA1 Mutant Mice
Video demonstrates the ability of the P2Y12 antagonist cangrelor (30-μg/kg bolus; 4-μg/kg/h infusion) to significantly reduce the ability of platelets from a neonatal cardiac patient from supporting thrombus growth without preventing core formation. In contrast, administration of the integrin αIIbβ3 blocker abciximab (0.25-μg/kg bolus; 0.125-μg/kg/min infusion) nearly abrogates platelet–vessel wall interactions, thereby preventing all aspects of thrombus formation (see Figure 6). Thrombus formation before administration of cangrelor is shown for comparison in the same animal. A new von Willebrand factor (VWF)HA1 mouse was used to assess the effects of abciximab.
P2Y12 surface expression and Response to ADP
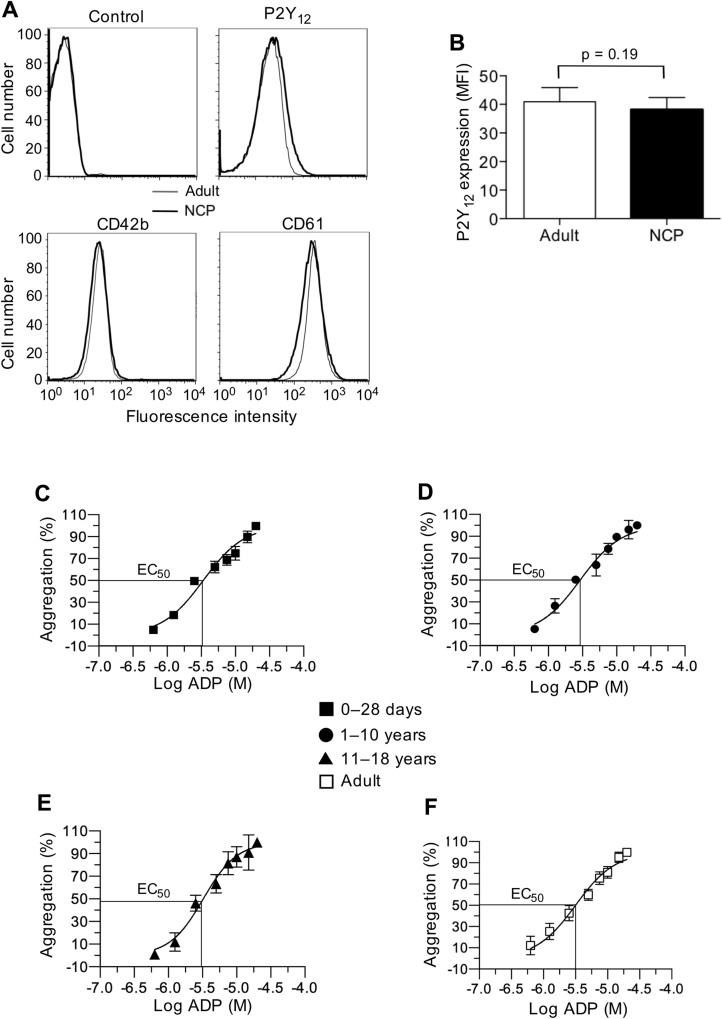
We next evaluated P2Y12 expression levels and function by using purified platelets from neonatal cardiac patients. Compared with platelets from adults, platelets from the neonatal cardiac patients that underwent flow cytometric analysis revealed no discernible difference in P2Y12 surface expression (Figures 4A and 4B), with mean fluorescence intensities of 38.1 ± 1.1 for neonates compared with 40.9 ± 2.4 for healthy adults (p = 0.19). To directly compare P2Y12 signaling, ADP dose–response curves were generated, and EC50 values (concentration of ADP that gives half-maximal response) were calculated by using isolated platelets in fibrinogen-supplemented buffer. By using purified platelets and adding back a known quantity of fibrinogen and calcium, this method avoided issues related to donor variation in fibrinogen levels and low levels of ionized calcium associated with use of citrate as an anticoagulant. Figures 4C to 4F show percent platelet aggregation as a function of ADP concentration. Calculated EC50 values were not significantly different for all the age groups tested, with values ranging from 2.9 ± 0.1 μmol/l to 3.4 ± 0.3 μmol/l (p = 0.42, 1-way analysis of variance model) (Table 2).
Figure 4.
P2Y12 Surface Expression and ADP Response
(A) Representative flow cytometry histograms of antibody staining for the P2Y12 receptor on resting platelets isolated from neonatal cardiac patients (NCP) versus healthy adults. Histograms of antibody staining for the human GPIbα (CD42b) and integrin αIIbβ3 (CD61) are shown for comparison. (B) Mean fluorescence intensities (MFI) of P2Y12. Results are presented in absolute arbitrary units. (C to F) Concentration response curves for the determination of EC50 (concentration of a drug that gives half-maximal response) values for adenosine diphosphate (ADP) as measured by light transmission aggregometry using purified platelets from neonatal and pediatric patients with congenital heart disease or healthy adult volunteers in the presence of fibrinogen (mean ± SEM). Concentrations of agonist are plotted in log form, and all data were normalized to the maximal concentration of ADP (20 μM). Data represent the mean ± SEM. n = 12 (age 0 to 28 days); n = 7 (age 1 to 10 years); n = 7 (age 11 to 18 years); n = 12 for adults. Statistical significance determined by using the Mann-Whitney U test.
Table 2.
ADP EC50 and Cangrelor IC50 Values
| Age 0–28 Days | Age 1–10 Years | Age 11–18 Years | Adult | |
|---|---|---|---|---|
| EC50 ± SEM, μmol/l | 3.4 ± 0.3 | 2.9 ± 0.1 | 3.2 ± 0.2 | 3.2 ± 0.2 |
| IC50 ± SEM,∗ nmol/l | 0.83 ± 0.1 | 0.79 ± 0.2 | 0.85 ± 0.2 | 0.82 ± 0.3 |
| IC50 ± SEM,† nmol/l | 0.72 ± 0.3 | 0.68 ± 0.3 | 0.76 ± 0.2 | 0.66 ± 0.3 |
Values are mean ± SD. One-way analysis of variance was performed to test significance among curve fits for multiple datasets.
EC50 = the concentration of a drug that gives half-maximal response; IC50 = the concentration of an inhibitor where the response (or binding) is reduced by one-half.
20 μmol/l of adenosine diphosphate (ADP) (p = 0.61).
5 μmol/l of ADP (p = 0.99).
Effect of cangrelor on platelet aggregation
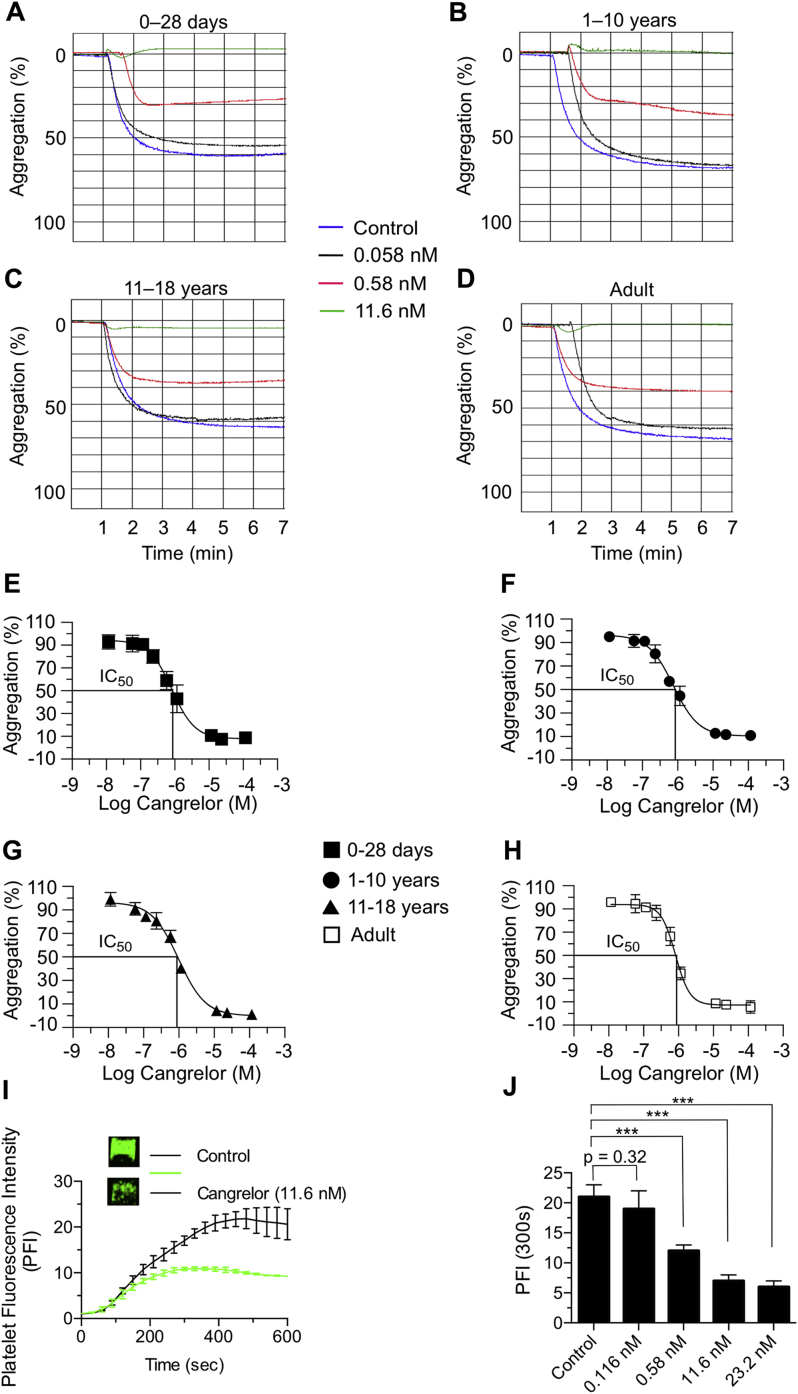
Using the identical assay system, we compared the effects of cangrelor on platelet aggregation induced by low and high concentrations of ADP (5 μmol/l vs. 20 μmol/l) and then calculated IC50 values (concentration of inhibitor where the response to ADP is reduced by one-half). The results indicate that the amount of cangrelor needed to achieve half-maximal inhibition at either concentration of ADP were similar for neonates, older populations of patients with CHD, and healthy adults (p = 0.99 for 5-μmol/l ADP and p = 0.61 for 20-μmol/l ADP based on one-way analysis of variance model) (Figures 5A to 5H, Table 2, Supplemental Figure 1).
Figure 5.
Inhibition of ADP-Induced Platelet Aggregation With Cangrelor
(A to D) Representative tracings of adenosine diphosphate (ADP) (20 μM)-induced aggregation of platelets isolated from neonatal and pediatric patients with congenital heart disease or healthy adult volunteers in the presence of indicated concentrations of cangrelor. (E to H) Concentration response curves for determination of IC50 values (the concentration of an inhibitor where the response [or binding] is reduced by one-half) for cangrelor (20-μM ADP) as measured by light transmission aggregometry. Concentrations of cangrelor are plotted in log form, and all data were normalized to aggregation in the absence of drug. n = 12 (age 0 to 28 days); n = 7 (age 1 to 10 years); n = 7 (age 11 to 18 years); n = 12 for adults. (I) Representative tracing of fluorescently labeled platelet accumulation on surface-immobilized collagen using whole blood from neonates with single-ventricle physiology and a multi-well microfluidic flow device (n = 5; 40 separate clotting events at 100 s−1). Insert shows platelet deposition at t = 300 s in the absence or presence of cangrelor (11.6 nM). (J) Mean platelet fluorescence intensities at t = 300 s after the addition of cangrelor to whole blood collected from the patients described in I. Data represent mean ± SEM. ***p < 0.001 for drug versus no treatment according to the Mann-Whitney U test. PFI = platelet fluorescence intensity.
To demonstrate that similar concentrations of cangrelor can reduce platelet aggregation when added directly to whole blood samples from neonatal cardiac patients, a low-volume, multi-well microfluidic device was used to assess platelet accumulation on surface-immobilized collagen. In this case, collagen not only serves as a binding surface for plasma VWF but also activates platelets through engagement of glycoprotein VI (GPVI) (29). In addition, the highly potent and selective inhibitor of thrombin PPACK was used as an anticoagulant to ensure an optimal concentration of extracellular cations needed for platelet integrin function (30). In each experiment, 8 simultaneously forming thrombi per device were imaged in real time. Fluorescently labeled platelets were observed to accumulate only at the site of collagen exposure, with minimal nonspecific upstream or downstream adhesion (Figure 5I). Determination of surface fluorescence at 300 s (PFI300s) permitted estimation of the effectiveness of cangrelor in reducing thrombus formation. Consistent with LTA studies, cangrelor at concentrations of ≥0.58 nM impaired the ability of platelets from neonates with CHD to promote thrombus growth (Figure 5J).
Discussion
Thrombotic complications such as acute shunt occlusion remain a major source of morbidity and mortality in the early post-operative period for neonates with cyanotic CHD requiring palliation with a systemic-to-pulmonary artery conduit 5, 6. Cangrelor, a P2Y12 inhibitor approved by the U.S. Food and Drug Administration with pharmacological properties well suited for use in this at-risk population, may fill an important therapeutic niche by providing effective protection during a period of time when patients are most vulnerable to clot formation: before the administration of an oral antiplatelet agent. The present study was designed to not only assess the overall reactivity of platelets from neonates with CHD but also the in vivo efficacy of cangrelor by using a unique avatar mouse model.
Of considerable importance in providing optimal thromboprophylaxis to critically ill neonates, especially those with structural heart disease, is identification of an appropriate target. This approach has been problematic because neonatal platelets are believed to be relatively hyporeactive as evidenced by their reduced response to common agonists, resulting in impaired granule secretion, aggregation, and expression of surface proteins compared with adult platelets 31, 32. However, this unique functional signature is based primarily on samples derived from cord blood and has never been verified in vivo. Interestingly, the observed hyporeactivity seems to be transient in nature, as several ex vivo studies have reported normalization of neonatal platelet function by 2 weeks of life 33, 34. This finding is particularly important because the mean age of neonatal patients enrolled in our study was 11.7 days, with the majority undergoing surgical repair/palliation at the time of blood sampling. Indeed, results support this concept as the P2Y12-signaling pathway is robust in platelets from this patient population, contributing significantly to in vivo thrombus formation. In particular, cangrelor not only prevented platelets isolated from neonates with CHD from supporting clot growth and vessel occlusion but also those from older patients with CHD and from healthy adults. It did not, however, completely abrogate platelet accumulation at sites of arterial injury as observed for the integrin αIIbβ3 inhibitor abciximab. Consistent with this observation is the finding that there is a significantly greater separation between antithrombotic effect and increase in bleeding time for cangrelor compared with inhibitors of integrin αIIbβ3 function (35). From a mechanistic standpoint, P2Y12 antagonists are known to primarily affect the formation of the outer thrombus shell and not its inner core, providing a rationale for the clinical observations that αIIbβ3 blockade is associated with the highest rate of bleeding complications (Figure 6) (36).
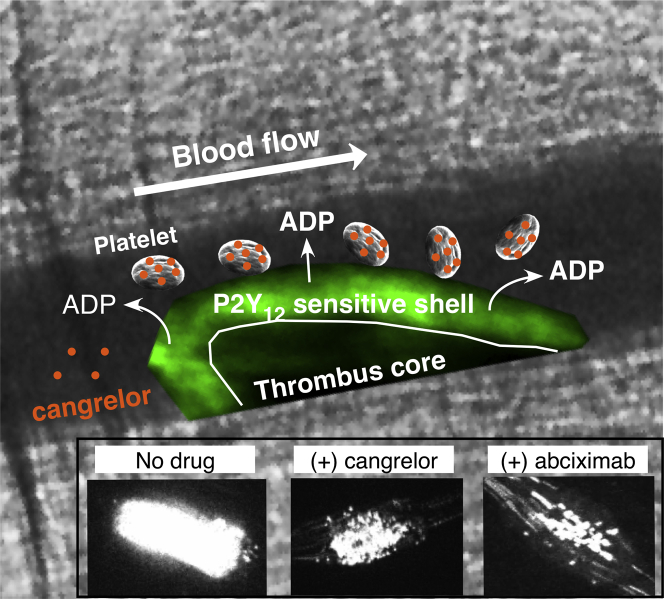
Figure 6.
Cangrelor Limits Thrombus Outer Shell Formation
An arterial thrombus is composed of distinct regions based on the primary mechanism of platelet activation (28). Thrombin is believed to drive platelet activation in the early phase of thrombus formation, resulting in a tightly packed core region (dark area) that limits its diffusion to the surface exposed to flowing blood. Further growth is reliant on adenosine diphosphate (ADP) release and thromboxane A2 generation by the outer layer of adherent platelets, enabling the recruitment of additional platelets that form the less dense shell region (green area). Cangrelor (orange dots) primarily targets the outer shell by blocking P2Y12 receptor-mediated platelet activation (inset). Both the core and outer shell formation are critically reliant on integrin αIIbβ3-mediated platelet adhesion as blockade with abciximab nearly abrogates thrombus formation (inset).
How well do our in vivo results compare with standard assays and microfluidic-based technologies currently used to assess P2Y12 receptor function? We were unable to discern any significant differences in platelet response to either ADP-induced aggregation or cangrelor-mediated P2Y12 receptor blockade for neonates and older pediatric patients with CHD compared with healthy adults in ex vivo studies. In fact, EC50 and IC50 values, respectively, were nearly identical for all age groups tested as determined by LTA using purified platelets. This outcome was further confirmed in microfluidic studies that evaluated the effects of cangrelor on thrombus generation triggered by surface-immobilized collagen. Despite the use of whole blood, cangrelor was able to limit thrombus growth at submicromolar drug concentrations as observed in LTA. Of note, a 20-fold smaller blood sample was required to obtain these results, which is of considerable significance due to the limited amount of blood that can be safely withdrawn from critically ill neonatal patients. Thus, there is much potential for such devices in serving as a drug efficacy biomarker in both preclinical and clinical trials involving this patient population.
Based on our findings, it was somewhat surprising that a previous clinical trial evaluating the P2Y12 inhibitor clopidogrel in infants with CHD undergoing palliation with a systemic-to-pulmonary artery shunt failed to show any significant reduction in either mortality from any cause or shunt-related morbidity (14). Clopidogrel, in combination with aspirin therapy, remains a cornerstone in the management of acute coronary syndromes and in patients undergoing PCI with stent placement. Possible explanations for the lack of drug efficacy may include the concomitant use of aspirin therapy in the majority of patients (>80%) and the wide variation in the degree of inhibition of platelet aggregation in response to clopidogrel treatment. In the latter case, this outcome may reflect patient variability in conversion of the prodrug to its active component due to maturational and/or genetic variations of the cytochrome P450 system, erratic drug absorption, and/or a need for greater inhibition of P2Y12 activity than initially anticipated. Clearly, these issues underscore the need for antiplatelet agents such as cangrelor that possess pharmacological properties well suited for critically ill neonates with CHD who require thromboprophylaxis.
Study limitations
A clinical trial will ultimately be required to determine the PK and PD properties of cangrelor, as well as to assess its safety and efficacy in preventing shunt thrombosis in the immediate post-operative period.
Conclusions
Platelets isolated from neonatal patients with CHD at the time of surgical repair/palliation have a robust response to ADP and are as amenable to P2Y12 inhibition with cangrelor as their adult counterparts. Moreover, our findings seem to be independent of age and type of cardiac lesion. Unique to this study was our ability to establish the in vivo efficacy of cangrelor by using an avatar mouse model that permits real-time evaluation of human platelet interactions with the injured vessel wall, yielding a biological response consistent with P2Y12 inhibition: disruption of thrombus shell formation. We also illustrated the value of a microfluidic device that can serve as a PD biomarker, requiring significantly less blood to assess changes in platelet reactivity than LTA. This multi-analytic approach will be used in the upcoming clinical trial at our institution that will assess the PK and PD properties of cangrelor in neonatal patients with CHD requiring palliation with a systemic-to-pulmonary artery shunt (NCT02765633).
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Neonates with cyanotic CHD requiring primary surgical palliation with a systemic-to-pulmonary shunt are at significant risk for thromboembolic events. Early initiation of thromboprophylaxis is believed to be essential for improving outcomes. We demonstrate that P2Y12 receptor signaling is robust in platelets from neonatal cardiac patients at the time of surgical repair/palliation and plays a significant role in supporting in vivo thrombus formation. Consistent with the biological role of this receptor was the ability of cangrelor to limit thrombus growth but not abolish platelet accrual at sites of arterial injury. This is of considerable clinical significance as administration of this antiplatelet agent to neonatal patients in the immediate post-operative period may prevent shunt thrombosis while preserving hemostasis.
TRANSLATIONAL OUTLOOK: A prospective, open-label, single-arm, PK/PD, and safety trial in neonatal subjects at risk of thrombosis is underway. This includes 4 cohorts of post-operative patients requiring palliation with a systemic-to-pulmonary shunt who will receive a continuous infusion of cangrelor (0.5, 1, 2, or 4 μg/kg) for 1 h, followed by blood sampling to establish drug clearance mechanisms and its metabolites. PD studies will include LTA and microfluidic assays (as described here) to determine the extent of inhibition of platelet function and time to recovery. Adverse events of special interest will include those related to perturbations in hemostasis.
Footnotes
This study was supported by grants from the National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD081281-01), the American Heart Association (16CSA28260000), and The Medicines Company. Two authors (Dr. Prats and Ms. Evans) are employed by the company whose product was studied in the present research. Dr. Prats has received consulting fees from the current sponsor of the study product (cangrelor [Chiesi USA, Inc.]). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
References
- 1.Manlhiot C., Menjak I.B., Brandão L.R. Risk, clinical features, and outcomes of thrombosis associated with pediatric cardiac surgery. Circulation. 2011;124:1511–1519. doi: 10.1161/CIRCULATIONAHA.110.006304. [DOI] [PubMed] [Google Scholar]
- 2.Reed R.C., Rutledge J.C. Laboratory and clinical predictors of thrombosis and hemorrhage in 29 pediatric extracorporeal membrane oxygenation nonsurvivors. Pediat Dev Pathol. 2010;13:385–392. doi: 10.2350/09-09-0704-OA.1. [DOI] [PubMed] [Google Scholar]
- 3.Monagle P. Thrombosis in children with BT shunts, Glenns and Fontans. Prog Pediatr Cardiol. 2005;21:17–21. [Google Scholar]
- 4.Fenton K.N., Siewers R.D., Rebovich B. Interim mortality in infants with systemic-to pulmonary artery shunts. Ann Thorac Surg. 2003;76:152–156. doi: 10.1016/s0003-4975(03)00168-1. [DOI] [PubMed] [Google Scholar]
- 5.Petrucci O., O'Brien S.M., Jacobs M.L. Risk factors for mortality and morbidity after the neonatal Blalock-Taussig shunt procedure. Ann Thorac Surg. 2011;92:642–651. doi: 10.1016/j.athoracsur.2011.02.030. [DOI] [PubMed] [Google Scholar]
- 6.Heidari-Bateni G., Norouzi S., Hall M. Defining the best practice patterns for the neonatal systemic-to-pulmonary artery shunt procedure. J Thorac Cardiovasc Surg. 2014;147:869–873. doi: 10.1016/j.jtcvs.2013.10.063. [DOI] [PubMed] [Google Scholar]
- 7.Motz R., Wessel A., Ruschewski W. Reduced frequency of occlusion of aorto-pulmonary shunts in infants receiving aspirin. Cardiol Young. 1999;9:474–477. doi: 10.1017/s1047951100005370. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal A., Firdouse M., Brar N. Incidence and management of thrombotic and thromboembolic complications following the Norwood procedure: a systematic review. Clin Appl Thromb Hemost. 2016 Nov 21 doi: 10.1177/1076029616679506. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Mir A., Frank S., Journeycake J. Aspirin resistance in single-ventricle physiology: aspirin prophylaxis is not adequate to inhibit platelets in the immediate postoperative period. Ann Thorac Surg. 2015;99:2158–2164. doi: 10.1016/j.athoracsur.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 10.Steinhubl S.R., Berger P.B., Mann J.T., 3rd Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288:2411–2420. doi: 10.1001/jama.288.19.2411. [DOI] [PubMed] [Google Scholar]
- 11.Gurbel P.A., Bliden K.P., Hiatt B.L. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation. 2004;107:2908–2913. doi: 10.1161/01.CIR.0000072771.11429.83. [DOI] [PubMed] [Google Scholar]
- 12.Dorsam R.T., Kunapuli S.P. Central role of the P2Y12 receptor in platelet activation. J Clin Invest. 2004;113:340–345. doi: 10.1172/JCI20986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franchi F., Angiolillo D.J. Novel antiplatelet agents in acute coronary syndrome. Nat Rev Cardiol. 2015;12:30–47. doi: 10.1038/nrcardio.2014.156. [DOI] [PubMed] [Google Scholar]
- 14.Wessel D.L., Berger F., Li J.S. Clopidogrel in infants with systemic-to-pulmonary-artery shunts. N Engl J Med. 2013;368:2377–2384. doi: 10.1056/NEJMoa1114588. [DOI] [PubMed] [Google Scholar]
- 15.Celestin C., Guillot M., Ross-Ascuitto N. Computational fluid dynamics characterization of blood flow in central aorta to pulmonary artery connections: importance of shunt angulation as a determinant of shear stress-induced thrombosis. Pediatr Cardiol. 2015;36:600–615. doi: 10.1007/s00246-014-1055-7. [DOI] [PubMed] [Google Scholar]
- 16.Li J.S., Yow E., Berezny K.Y. Dosing of clopidogrel for platelet inhibition in infants and young children: primary results of the Platelet Inhibition in Children On cLOpidogrel (PICOLO) trial. Circulation. 2008;117:553–559. doi: 10.1161/CIRCULATIONAHA.107.715821. [DOI] [PubMed] [Google Scholar]
- 17.Akers W.S., Oh J.J., Oestreich J.H. Pharmacokinetics and pharmacodynamics of a bolus and infusion of cangrelor: a direct, parenteral P2Y12 receptor antagonist. J Clin Pharmacol. 2010;50:27–35. doi: 10.1177/0091270009344986. [DOI] [PubMed] [Google Scholar]
- 18.Van Giezen J.J., Humphries R.G. Preclinical and clinical studies with selective reversible direct P2Y12 antagonists. Semin Thromb Hemost. 2005;31:195–204. doi: 10.1055/s-2005-869525. [DOI] [PubMed] [Google Scholar]
- 19.Bhatt D.L., Stone G.W., Mahaffey K.W. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N Engl J Med. 2013;368:1303–1313. doi: 10.1056/NEJMoa1300815. [DOI] [PubMed] [Google Scholar]
- 20.Angiolillo D.J., Firstenberg M.S., Price M.J. Bridging antiplatelet therapy with cangrelor in patients undergoing cardiac surgery: a randomized controlled trial. JAMA. 2012;307:265–274. doi: 10.1001/jama.2011.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kozik D.J., Tweddell J.S. Characterizing the inflammatory response to cardiopulmonary bypass in children. Ann Thorac Surg. 2006;81:S2347–S2354. doi: 10.1016/j.athoracsur.2006.02.073. [DOI] [PubMed] [Google Scholar]
- 22.Magallon J., Chen J.C., Rabbani L. Humanized mouse model of thrombosis is predictive of the clinical efficacy of antiplatelet agents. Circulation. 2011;123:319–326. doi: 10.1161/CIRCULATIONAHA.110.951970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J.C., Zhou H., Diacovo A. Exploiting the kinetic interplay between the GPIbα–VWF binding interfaces to regulate hemostasis and thrombosis. Blood. 2014;124:3799–3807. doi: 10.1182/blood-2014-04-569392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li R., Diamond S.L. Detection of platelet sensitivity to inhibitors of COX-1, P2Y1, and P2Y12 using a whole blood microfluidic flow assay. Thrombosis Res. 2013;133:203–210. doi: 10.1016/j.thromres.2013.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coller B.S., Shattil S.J. The GPIIb/IIIa (integrin alphaIIbbeta3) odyssey: a technology-driven saga of a receptor with twists, turns, and even a bend. Blood. 2008;112:3011–3025. doi: 10.1182/blood-2008-06-077891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doggett T.A., Girdhar G., Lawshe A. Selectin-like kinetics and biomechanics promote rapid platelet adhesion in flow: the GPIbα-vWF tether bond. Biophys J. 2002;83:194–205. doi: 10.1016/S0006-3495(02)75161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrington R.A., Stone G.W., McNulty S. Platelet inhibition with cangrelor in patients undergoing PCI. N Engl J Med. 2009;361:2318–2329. doi: 10.1056/NEJMoa0908628. [DOI] [PubMed] [Google Scholar]
- 28.Stalker T.J., Traxler E.A., Wu J. Hierarchical organization in the hemostatic response and its relationship to the platelet-signaling network. Blood. 2013;121:1875–1885. doi: 10.1182/blood-2012-09-457739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nieswandt B., Watson S.P. Platelet-collagen interaction: is GPVI the central receptor? Blood. 2003;102:449–461. doi: 10.1182/blood-2002-12-3882. [DOI] [PubMed] [Google Scholar]
- 30.Marciniak S.J., Jr., Jordan R.E., Mascelli M.A. Effect of Ca2+ chelation on the platelet inhibitory ability of the GPIIb/IIIa antagonists abciximab, eptifibatide and tirofiban. Thromb Haemost. 2001;85:539–543. [PubMed] [Google Scholar]
- 31.Rajasekhar D., Kestin A.S., Bednarek F.J. Neonatal platelets are less reactive than adult platelets to physiological agonists in whole blood. Thromb Haemost. 1994;72:957–963. [PubMed] [Google Scholar]
- 32.Gader A.M., Bahakim H., Jabbar F.A. Dose-response aggregometry in maternal/neonatal platelets. Thromb Haemost. 1988;60:314–318. [PubMed] [Google Scholar]
- 33.Tanindi S., Kürekçi A.E., Köseoglu V., Kurt M., Özcan O. The normalization period of platelet aggregation in newborns. Thromb Res. 1995;80:57–62. doi: 10.1016/0049-3848(95)00150-p. [DOI] [PubMed] [Google Scholar]
- 34.Sitaru A.G., Holzhauer S., Speer C.P. Neonatal platelets from cord blood and peripheral blood. Platelets. 2005;16:203–210. doi: 10.1080/09537100400016862. [DOI] [PubMed] [Google Scholar]
- 35.Ingall A.H., Dixon J., Bailey A. Antagonists of the platelet P2T receptor: a novel approach to antithrombotic therapy. J Med Chem. 1999;42:213–220. doi: 10.1021/jm981072s. [DOI] [PubMed] [Google Scholar]
- 36.Serebruany V.L., Malinin A.I., Eisert R.M. Risk of bleeding complications with antiplatelet agents: meta-analysis of 338,191 patients enrolled in 50 randomized controlled trials. Am J Hematol. 2004;75:40–47. doi: 10.1002/ajh.10451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.