There is an urgent unmet need for better treatment of atrial fibrillation (AF) given its strong impact on morbidity and mortality and the expected increase in AF prevalence with the ageing of the population.1, 2 Current AF management involves antithrombotic therapy to reduce the risk of stroke and normalization of the ventricular response rate (“rate control”) or restoration and maintenance of normal sinus rhythm (“rhythm control”).3, 4 Although numerous clinical trials have established that both rate and rhythm control produce similar outcomes, rhythm control is often attempted to reduce AF symptoms.3, 5 Antiarrhythmic drugs and catheter ablation are the most commonly employed approaches for rhythm-control therapy. Although ablation is generally more effective in maintaining normal sinus rhythm than antiarrhythmic drugs, it is associated with a significant risk for adverse events3, 4 and is not an option for every AF patient, particularly in light of the high costs, the need of specialized skills and the expected increase in AF prevalence. Moreover, a large fraction the patients that undergo AF ablation receive additional subsequent treatment with antiarrhythmic drugs.3 Thus, antiarrhythmic drugs still have a major impact on AF management. However, currently available antiarrhythmic drugs have limited efficacy, particularly in longer-lasting forms of AF, and a substantial risk of adverse effects, including ventricular proarrhythmia, which are likely in large part due to their development in the absence of a detailed understanding of AF mechanisms.6, 7
Pharmacological AF therapy generally targets the two main arrhythmogenic mechanisms: ectopic activity and reentry (Figure 1A).8 Ectopic activity is inhibited by reducing atrial excitability (e.g., using class I antiarrhythmic drugs blocking the fast Na+-current), and reentry is suppressed by prolonging atrial effective refractory period (ERP; e.g., with class III antiarrhythmic drugs inhibiting K+-currents). In recent years, several new classes of antiarrhythmic drugs have been developed based on new insights into the molecular and cellular basis of these arrhythmogenic mechanisms.7, 8 In particular, significant attention has been paid to atrial-selective antiarrhythmic drugs, which would be expected to have a lower risk of ventricular proarrhythmia.6, 9 Inhibition of the atrial-specific ultra-rapid delayed rectifier and acetylcholine-activated inward-rectifier K+-currents (IKur and IK,ACh, respectively) were among the first of these approaches, but most compounds targeting IKur and IK,ACh have been discontinued after unsuccessful initial clinical trials.6, 9 Recently, small-conductance Ca2+-activated K+-channels (SK-channels) have been proposed as alternative antiarrhythmic targets for the treatment of AF. Under physiological conditions, functional SK-channels are predominantly present in the atria and contribute to repolarization and stabilization of the atrial resting membrane potential. Besides the bee-venom toxin apamin, a highly selective SK-channel blocker often used to identify the SK current experimentally, several compounds with different mechanisms of SK-channel inhibition have been developed. For example, NS8593 modulates the sensitivity of the calmodulin-formed Ca2+-sensor, whereas UCL1684 and ICAGEN block the SK-channel pore (Figure 1B).9 These SK-channel inhibitors prolong repolarization in human atrial samples10 and have been shown to increase atrial ERP and reduce AF inducibility and/or duration in various animal models (reviewed in El Haou et al.11). However, these animal studies were limited to acute forms of AF with lower complexity than that usually detected in AF patients.12
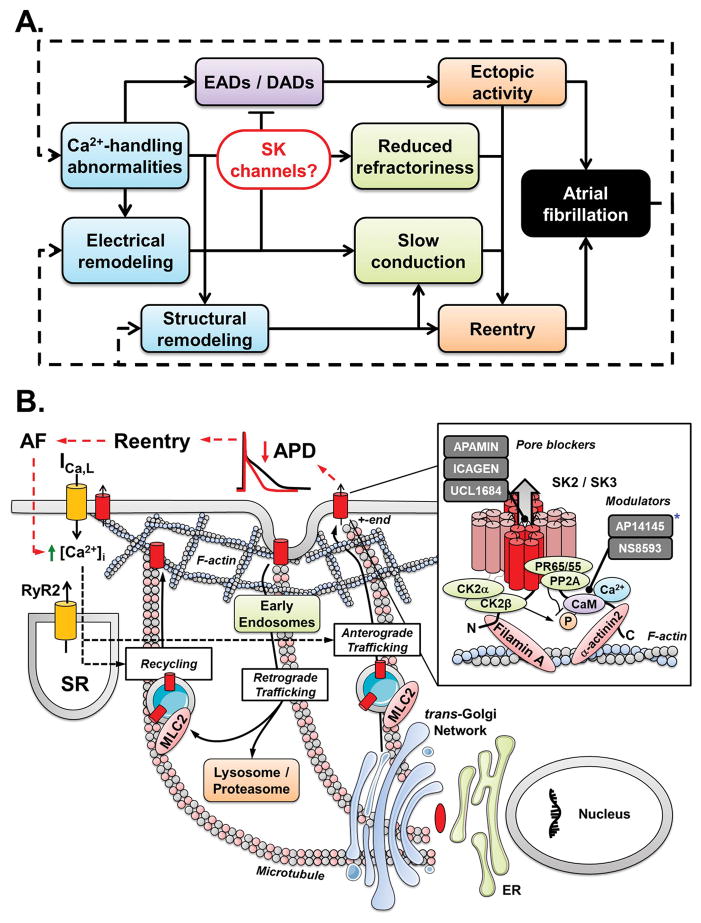
Figure 1.
A. Fundamental mechanisms of atrial fibrillation (AF) and the central role for small-conductance Ca2+-activated K+ (SK) channels linking Ca2+-handling abnormalities and repolarization disturbances. B. Complexity of atrial cardiomyocyte SK-channel regulation involving Ca2+-dependent trafficking and phosphorylation-modulated Ca2+-dependent gating of SK-channels within the macromolecular multiprotein complex.20 The trafficking (anterograde and retrograde) of SK-channels is dynamic and depends on interactions with microtubule and F-actin cytoskeletal proteins. SK2-channels recycle quickly between the plasma membrane and early (recycling) endosomes via a mechanism involving α-actinin2 and filamin A. Myosin light chain 2 (MLC2) is also required for membrane trafficking and localization of SK2-channels. SK-channels are degraded via the lysosome/proteasome system. The precise trafficking pathways of SK-channels in the human atrium need further determination. The Ca2+-dependent gating of SK-channels is mediated by calmodulin (CaM). SK2- and SK3-channels are organized in macromolecular multiprotein complexes including CaM, protein kinases (e.g., casein kinase 2α and 2β; CK2α and CK2β, respectively) and phosphatases (e.g., type 2a; PP2A). CK2 and PP2A alter the Ca2+ sensitivity of SK-channels by phosphorylation (which decreases Ca2+-sensitivity and reduces ISK) and dephosphorylation (opposite effects) of SK-associated CaM at threonine-80. Whether Ca2+-dependent gating of atrial SK channels is similarly regulated by dynamic CaM-mediated phosphorylation in vivo is not established. Grey squares in inset show available SK-channel blockers grouped by their mechanism of action (pore block vs. modulation of Ca2+-dependent gating). * The compound AP14145 presented in the study by Diness et al.13 in the present issue of the journal is listed as modulator based on its structural similarity to NS8593. DADs, delayed afterdepolarisations; RyR2, ryanodine-receptor channels type-2; ICa,L, L-type Ca2+-current; APD, action potential duration; SR, sarcoplasmic reticulum; PR65/55, regulatory PP2A subunit.
In the present issue of Circulation Arrhythmia & Electrophysiology, Diness et al.13 describe the antiarrhythmic effects of a novel, reasonably selective SK-channel blocker (AP14145; with a 10-fold difference between the IC50 for SK-channels and IK,ACh) in pigs. AP14145 prolongs atrial ERP and reduces the duration of acutely induced AF in pigs subjected to one week of atrial tachycardia remodeling. These results were similar to those obtained with vernakalant, a multichannel blocker affecting, among others, IKur and the fast Na+-current, which is approved for pharmacological cardioversion of recent-onset AF in patients without severe heart failure in Europe.3 Moreover, using long-lasting rapid atrial pacing protocols that were continued until AF could no longer be terminated by vernakalant, Diness et al.13 show that SK-channel inhibition is able to terminate vernakalant-resistant AF. This important finding suggests that SK-channel inhibition may be effective in more persistent, drug-refractory forms of AF. Moreover, AP14145 was able to prevent re-induction of AF under these conditions, hinting towards a potential future use for long-term rhythm control therapy.
The work by Diness et al. adds to the ongoing discussion about the antiarrhythmic potential of SK-channel inhibition. Indeed, SK-channels appear to represent a critical feedback mechanism, linking atrial cardiomyocyte Ca2+-handling and electrophysiology. Although SK-channel inhibition may prolong atrial ERP and inhibit reentrant forms of AF, it may simultaneously make the atrial cardiomyocyte more sensitive to Ca2+-dependent triggered activity (Figure 1A). Moreover, increased expression of SK-channels during cardiovascular disease may prevent excessive repolarization prolongation and associated early afterdepolarization (EAD)-mediated arrhythmias. In agreement, SK2-knockout mice have prolonged atrial repolarization and are vulnerable to EADs and AF induction,14 and SK-channel blockade (with apamin or UCL1684) causes delayed repolarization, alternans and wave-breaks, promoting arrhythmias in isolated dog left atrium.15 An SK-channel activator has also recently been shown to attenuate Ca2+-dependent arrhythmias in hypertrophic rat hearts by regulating SK-channels on the mitochondrial membrane, reducing mitochondrial reactive oxygen species production, whereas UCL1684 increased oxidative stress.16 Thus, inhibition of SK-channels may be both pro- and antiarrhythmic.
Moreover, the species- and disease-specific differences in the expression and regulation of SK-channel isoforms remain incompletely understood. At the mRNA level the KCNN2 gene encoding the SK2 isoform appears to be most abundantly expressed in the human heart,10, 17 whereas dogs predominantly express SK1.18 Although pigs appear to have an isoform expression pattern more similar to humans,13 little is known about the functional regulation of SK-channels, e.g., due to species-specific differences in intracellular Ca2+-handling. Moreover, even between different studies in human atrial samples, there is disagreement about the direction of disease-related changes in SK-channel expression and function, with both up- and downregulation of SK-channel expression and SK current amplitude being reported in AF patients compared to sinus rhythm controls.6, 9, 10 These discrepancies may be due to differences in patient population and employed methodology and suggest a dynamic disease-related remodeling of SK-channels. Most animal models are not able to fully recapitulate the complexity of AF observed in patients.7 In addition, SK2 and other SK-channel isoforms are also expressed in various other tissues including brain, liver, bladder and prostate,17 creating the potential for adverse extra-cardiac side effects. Indeed, all conscious pigs in the study by Diness et al. showed adverse effects requiring additional treatment.13
Importantly, mRNA or protein levels from tissue homogenates likely only provide limited information about the amount and composition of functional SK-channels in atrial cardiomyocytes. Indeed, intracellular Ca2+ not only regulates SK-channel gating, but also strongly promotes the trafficking of SK-channels to the plasma membrane (Figure 1B). Accordingly, short-term atrial burst-pacing, which leads to rapid cellular Ca2+-overload, accelerates trafficking of SK2-channel subunits to the plasma membrane and creates a proarrhythmic ERP shortening in dog pulmonary veins.19 Moreover, Ca2+-dependent SK-channel gating is also gradually fine-tuned by phosphorylation of threonine-80 of the Ca2+-sensor calmodulin, at least in neurons (Figure 1B).20 Thus, the importance of species-specific and disease-linked differences in subcellular Ca2+-handling, SK-channel trafficking pathways and Ca2+-dependent SK-channel gating highlight the clear need for a comprehensive characterization of SK-channel regulation and function in remodeled human atria to determine the subpopulations of AF patients that are most likely to benefit from SK-channel inhibition.
Taken together, the study by Diness et al.13 supports the idea that SK-channels are a critical link between triggered activity and reentry, and that SK-channel inhibition is a promising antiarrhythmic strategy for pharmacological conversion of AF. Moreover, although a lot of work still needs to be done, future derivatives of AP14145 with improved pharmacokinetic properties (current half-life ~24 minutes) and fewer adverse extra-cardiac effects or other SK-channel blockers should be tested in carefully selected patient groups, particularly in the first-in-man trial. Because SK-channel blockers appear to be the only class of antiarrhythmic drugs in development not yet investigated in patients, there remains hope that such compounds may provide the long-awaited breakthrough in the treatment of AF with antiarrhythmic drugs.
Acknowledgments
Sources of Funding: The authors’ work is supported by the Netherlands Organization for Scientific Research (ZonMW Veni 91616057 to J.H.), the National Institutes of Health (R01-HL131517 and R01-136389 to D.D), the DZHK (German Center for Cardiovascular Research, grants 81X2800108, 81X2800161, and 81X2800136 to D.D.) and the German Research Foundation (DFG, Do 769/4-1 to D.D.).
Footnotes
Disclosures: Dr. Dobrev is on the Scientific Advisory Board of OMEICOS and received speaker’s fees from Boston Scientific, Daiichi Sankyo and Servier. His laboratory executed a research contract for Omeicos. Dr. Heijman has no conflicts of interest to disclose.
References
- 1.Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014;114:1453–1468. doi: 10.1161/CIRCRESAHA.114.303211. [DOI] [PubMed] [Google Scholar]
- 2.Kirchhof P, Breithardt G, Bax J, et al. A roadmap to improve the quality of atrial fibrillation management: proceedings from the fifth Atrial Fibrillation Network/European Heart Rhythm Association consensus conference. Europace. 2016;18:37–50. doi: 10.1093/europace/euv304. [DOI] [PubMed] [Google Scholar]
- 3.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 4.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–267. doi: 10.1161/CIR.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piccini JP, Fauchier L. Rhythm control in atrial fibrillation. Lancet. 2016;388:829–840. doi: 10.1016/S0140-6736(16)31277-6. [DOI] [PubMed] [Google Scholar]
- 6.Heijman J, Ghezelbash S, Dobrev D. Investigational antiarrhythmic agents: promising drugs in early clinical development. Expert Opin Investig Drugs. 2017;26:897–907. doi: 10.1080/13543784.2017.1353601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heijman J, Algalarrondo V, Voigt N, Melka J, Wehrens XH, Dobrev D, Nattel S. The value of basic research insights into atrial fibrillation mechanisms as a guide to therapeutic innovation: a critical analysis. Cardiovasc Res. 2016;109:467–479. doi: 10.1093/cvr/cvv275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heijman J, Voigt N, Nattel S, Dobrev D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ Res. 2014;114:1483–1499. doi: 10.1161/CIRCRESAHA.114.302226. [DOI] [PubMed] [Google Scholar]
- 9.Ravens U, Odening KE. Atrial fibrillation: Therapeutic potential of atrial K+ channel blockers. Pharmacol Ther. 2017;176:13–21. doi: 10.1016/j.pharmthera.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Skibsbye L, Poulet C, Diness JG, et al. Small-conductance calcium-activated potassium (SK) channels contribute to action potential repolarization in human atria. Cardiovasc Res. 2014;103:156–167. doi: 10.1093/cvr/cvu121. [DOI] [PubMed] [Google Scholar]
- 11.El-Haou S, Ford JW, Milnes JT. Novel K+ Channel Targets in Atrial Fibrillation Drug Development - Where Are We? J Cardiovasc Pharmacol. 2015;66:412–431. doi: 10.1097/FJC.0000000000000277. [DOI] [PubMed] [Google Scholar]
- 12.Goette A, Kalman JM, Aguinaga L, et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Europace. 2016;18:1455–1490. doi: 10.1093/europace/euw161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diness JG, Skibsbye L, Vicens RS, et al. Termination of Vernakalant-Resistant Atrial Fibrillation by Inhibition of Small Conductance Ca2+-activated K+-channels in Pigs. Circ Arrhythm Electrophysiol. 2017;10:e005125. doi: 10.1161/CIRCEP.117.005125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li N, Timofeyev V, Tuteja D, et al. Ablation of a Ca2+-activated K+ channel (SK2 channel) results in action potential prolongation in atrial myocytes and atrial fibrillation. J Physiol. 2009;587:1087–1100. doi: 10.1113/jphysiol.2008.167718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsueh CH, Chang PC, Hsieh YC, Reher T, Chen PS, Lin SF. Proarrhythmic effect of blocking the small conductance calcium activated potassium channel in isolated canine left atrium. Heart Rhythm. 2013;10:891–898. doi: 10.1016/j.hrthm.2013.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim TY, Terentyeva R, Roder KH, et al. SK channel enhancers attenuate Ca2+-dependent arrhythmia in hypertrophic hearts by regulating mito-ROS-dependent oxidation and activity of RyR. Cardiovasc Res. 2017;113:343–353. doi: 10.1093/cvr/cvx005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen MX, Gorman SA, Benson B, Singh K, Hieble JP, Michel MC, Tate SN, Trezise DJ. Small and intermediate conductance Ca2+-activated K+ channels confer distinctive patterns of distribution in human tissues and differential cellular localisation in the colon and corpus cavernosum. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:602–615. doi: 10.1007/s00210-004-0934-5. [DOI] [PubMed] [Google Scholar]
- 18.Qi XY, Diness JG, Brundel BJ, et al. Role of small-conductance calcium-activated potassium channels in atrial electrophysiology and fibrillation in the dog. Circulation. 2014;129:430–440. doi: 10.1161/CIRCULATIONAHA.113.003019. [DOI] [PubMed] [Google Scholar]
- 19.Ozgen N, Dun W, Sosunov EA, Anyukhovsky EP, Hirose M, Duffy HS, Boyden PA, Rosen MR. Early electrical remodeling in rabbit pulmonary vein results from trafficking of intracellular SK2 channels to membrane sites. Cardiovasc Res. 2007;75:758–769. doi: 10.1016/j.cardiores.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bildl W, Strassmaier T, Thurm H, et al. Protein kinase CK2 is coassembled with small conductance Ca2+-activated K+ channels and regulates channel gating. Neuron. 2004;43:847–858. doi: 10.1016/j.neuron.2004.08.033. [DOI] [PubMed] [Google Scholar]