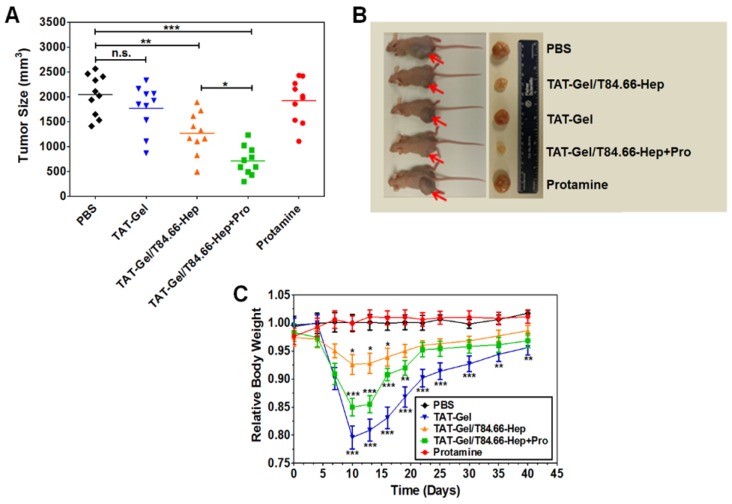
Fig 10.
In vivo proof-of-concept efficacy study for assessment of the feasibility of PTD-modified ATTEMPTS using LS174T xenograft tumor bearing mice. (A) Tumor volumes (mm3) at day 40 (40 days after tumor implantation). At day 3, mice were divided into 5 groups (N = 10) and treated with either: 1) PBS, 2) TAT-Gel, 3) TAT-Gel/T84.66-Hep, 4) “TAT-Gel/T84.66-Hep+Pro” or 5) protamine (a.k.a. Pro) for three times (at day 3, 6 and 9) via tail vein injection. (B) Representative mice and tumor images at day 40. (C) Relative average body weight change (%) of mice during efficacy study. “TAT-Gel/T84.66-Hep+Pro” treatment exerted significantly enhanced therapeutic effects (*P < 0.05), compared with TAT-Gel/T84.66-Hep treatment, yet induced higher toxicity. *P < 0.05, **P < 0.01 and ***P < 0.001 by 1-way ANOVA (Tukey's multiple comparison test as the post hoc test). (TAT-Gel: recombinant TAT-gelonin fusion chimera, T84.66-Hep: T84.66-heparin chemical conjugate, TAT-Gel/T84.66-Hep+Pro”: treatment with TAT-Gel/T84.66-Hep complex with protamine). Reproduced with permission from 55.