Scheme 3.
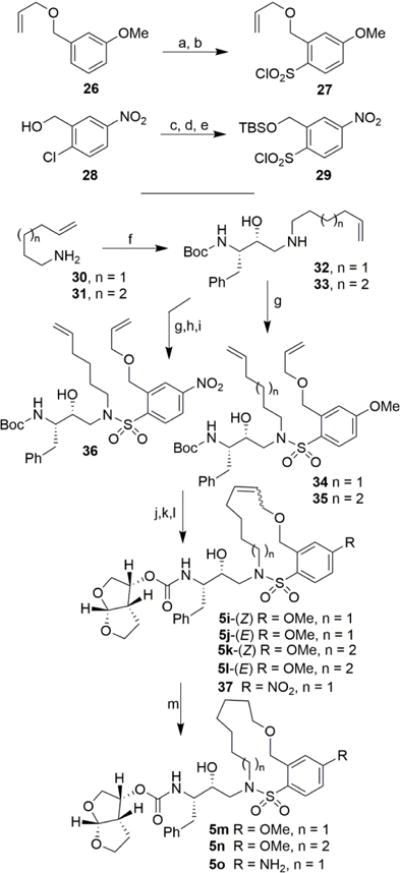
Reagents and conditions: (a) HSO3Cl, CH2Cl2, 0 °C, 20 min; (b) cyanuric chloride, Et3N, dry acetone, 23 °C to 60 °C, 24 h, 37% over 2 steps; (c) TBSCl, NaH (60% susp. in mineral oil), TBAI, dry THF, 0 °C, 1 h, 90%; (d) Na2S2 in EtOH, NaOH, EtOH, reflux, 2 h; (e) NCS, 2M HCl in MeCN, − 10 °C to 20 °C, 30 min, 35% over 2 steps; (f) 9, iPrOH, 56 °C, 14 h, 82% for n = 1, 63% for n = 2; (g) 27 or 29, aqueous NaHCO3, CH2Cl2, 23 °C, 18 h, 85–92%; (h) TBAF, dry THF, 0 °C, 15 min, 78%; (o) allyl-tert-butylcarbonate, Pd(PPh3)4, dry THF, 60 °C, 3 h; (j) Grubbs II, dry CH2Cl2, 40 °C, 3–6 h, 85–93%; (k) TFA, CH2Cl2, 23 °C, 3 h; (l) 14, DIPEA, MeCN, 23 °C, 8 days, 34–60%; (m) H2, 10% Pd/C, EtOAc, 23 °C, 12 h, 87–90%.
