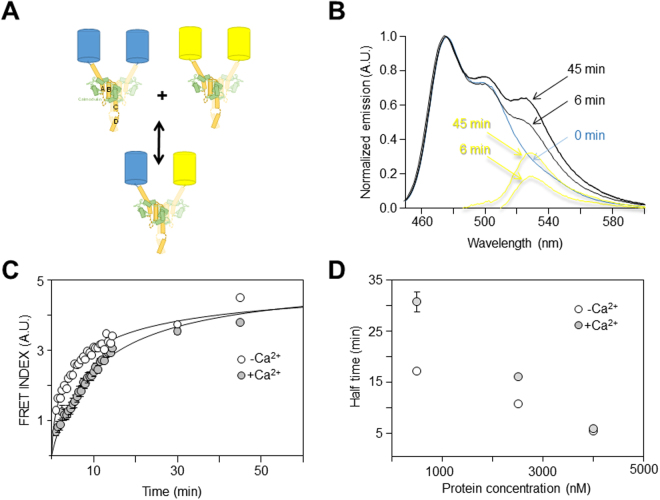
Figure 6.
The time-course of subunit exchange between FP-ABCD/CaM tetrameric complexes is affected by calcium. Two ABCD/CaM complexes with a fluorescent protein attached to the N-terminus (CFP, donor; YFP, acceptor) were purified and the development of FRET was monitored over time from equimolar mixtures. (A) Cartoon representing the experiment: the CFP-ABCD/CaM complex was mixed with YFP-ABCD/CaM, resulting in an exchange of proteins that led to the development of FRET. Only two subunits of the tetrameric complexes are drawn for clarity. (B) Normalized emission spectra of a mixture of 2.5 µM CFP-ABCD/CaM and 2.5 µM YFP-ABCD/CaM at different times. The yellow traces are the results of subtracting the normalized CFP emission spectra and isolating the emission of the acceptor (YFP). (C) Time course of the increase in the FRET index from a 2.5 µM CFP-ABCD/CaM and 2.5 µM YFP-ABCD/CaM mixture in the presence (gray circles) and absence (white circles) of Ca2+. Each trace represents the average of 3 experiments. (D) Relationship between the time to reach the half-maximal increase in the FRET index and the protein concentration in the presence (gray circles) and absence of Ca2+ (white circles). Each point represents the average of 3 or more experiments.