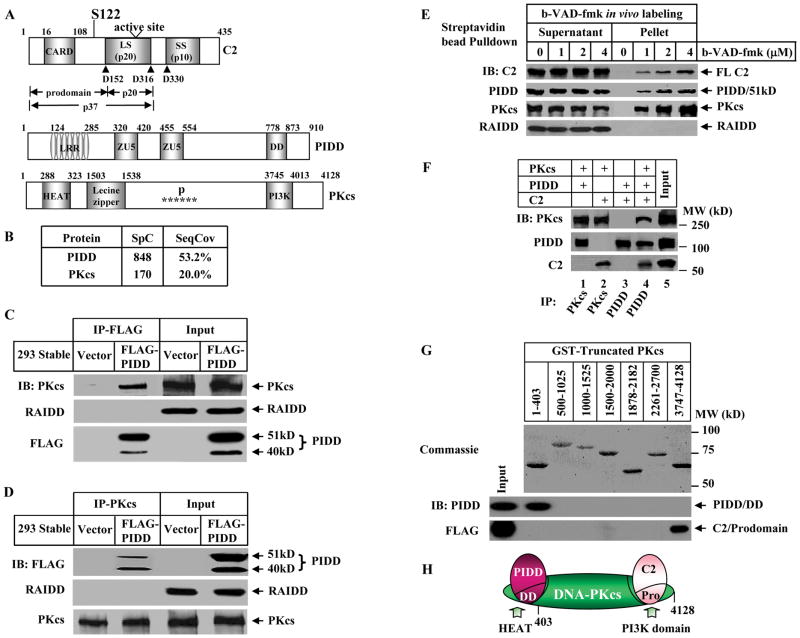
Figure 1. PIDD, DNA-PKcs, and Caspase-2 Form a Nuclear DNA-PKcs-PIDDosome.
(A)Domain organizations in human caspase-2 (C2), PIDD, and DNA-PKcs (PKcs). Arrowheads point to the three cleavage sites in caspase-2. LS: large subunit (p20); SS: small subunit (p10). Domains in PIDD shown are leucine rich repeat (LRR), ZU5 (ZO-1 and Unc5-like netrin receptors), and the death domain (DD). Domains in DNA-PKcs shown are a HEAT (huntingtin-elongation-A-subunit-TOR) repeat, clusters of phosphorylation sites (P and stars), and the catalytic PI-3 kinase domain (PI3K).
(B) DNA-PKcs is a major PIDD-interacting protein in cell nucleus identified by mass spectrometry. PIDD-interacting proteins were purified by immunoprecipitation (IP) from HEK293 cells stably expressing FLAG-PIDD. Shown are spectral counts (SpC) and sequence coverage (SeqCov) for PIDD and DNA-PKcs.
(C) Association of DNA-PKcs and PIDD. Nuclear extracts (50 μg) were prepared from the HEK293 cells described in (B) and HEK293 cells expressing an empty vector (Vector). PIDD was immunoprecipitated by anti-FLAG-M2 antibody and the IP samples were analyzed by immunoblotting (IB) against FLAG, DNA-PKcs and RAIDD.
(D)Identical nuclear extracts to (C) were immunoprecipitated by anti-DNA-PKcs antibody.
(E) In vivo association of endogenous caspase-2, DNA-PKcs, and PIDD in live HeLa cell nuclei. HeLa cells (2X107) were labeled with cell-permeable biotinylated-VAD-fmk (b-VAD-fmk) or DMSO (0) and the b-VAD-fmk-bound protein complex was isolated by streptavidin bead-pull down and immunoblotted with the indicated antibodies. Caspase-2 was full length (FL) detected by anti-caspase-2 antibody.
(F) In vitro direct binding of the three proteins. Recombinant PIDD and caspase-2 (purified from bacteria) and DNA-PKcs (purified from HeLa cell nuclei) were incubated each at 20 nM either in pairs or all three together. Protein bindings were examined by IP followed by IB.
(G)Mapping the interaction domains. GST-fused fragments of DNA-PKcs (top, stained by Coomassie Blue R-250) were incubated either with recombinant DD of PIDD or with the FLAG-tagged prodomain of caspase-2 followed by glutathione agarose pulldown. The protein interactions were detected by IB using anti-PIDD and anti-FLAG antibodies. Numbers on the top indicate the DNA-PKcs residues included in each GST-fused truncated DNA-PKcs proteins.
(H)Schematic of interactions. DNA-PKcs is the central component to bridge PIDD and caspase-2 in the complex. Pro: the prodomain of caspase-2.