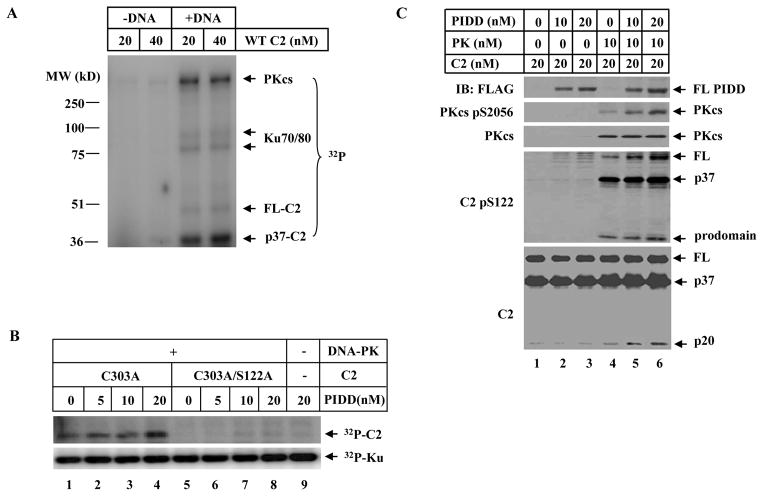
Figure 4. DNA-PK Directly Phosphorylates Caspase-2 In Vitro and PIDD Promotes the Kinase Activity of DNA-PKcs.
(A)DNA-PK directly phosphorylates caspase-2 in vitro. A standard in vitro DNA-PK protein kinase assay was performed with purified recombinant human wild-type caspase-2 in the presence or absence of sonicated salmon sperm DNA. The reaction products were resolved on SDS-PAGE and PhosphorImager analysis showed γ-[32P]-ATP incorporation into caspase-2, DNA-PKcs, and Ku70/80.
(B) PIDD enhances DNA-PKcs-dependent caspase-2 phosphorylation at the S122 site in vitro. Same in vitro DNA-PKcs reactions as in (A) were performed with purified recombinant human caspase-2 mutated at catalytic site (C303A) or double-mutated at both catalytic and phosphorylation sites (C303A/S122A) with purified recombinant PIDD added in some reactions, and the products were analyzed by PhosphorImager.
(C) PIDD activates DNA-PK kinase activity. DNA-PK kinase assays (cold, without γ-[32P]-ATP) were performed in the presence of 10mM ATP and the reaction products were analyzed by IB.