Fig. 2. Adoptive transfer of neoantigen-stimulated T cells inhibits growth of PDXs in vivo.
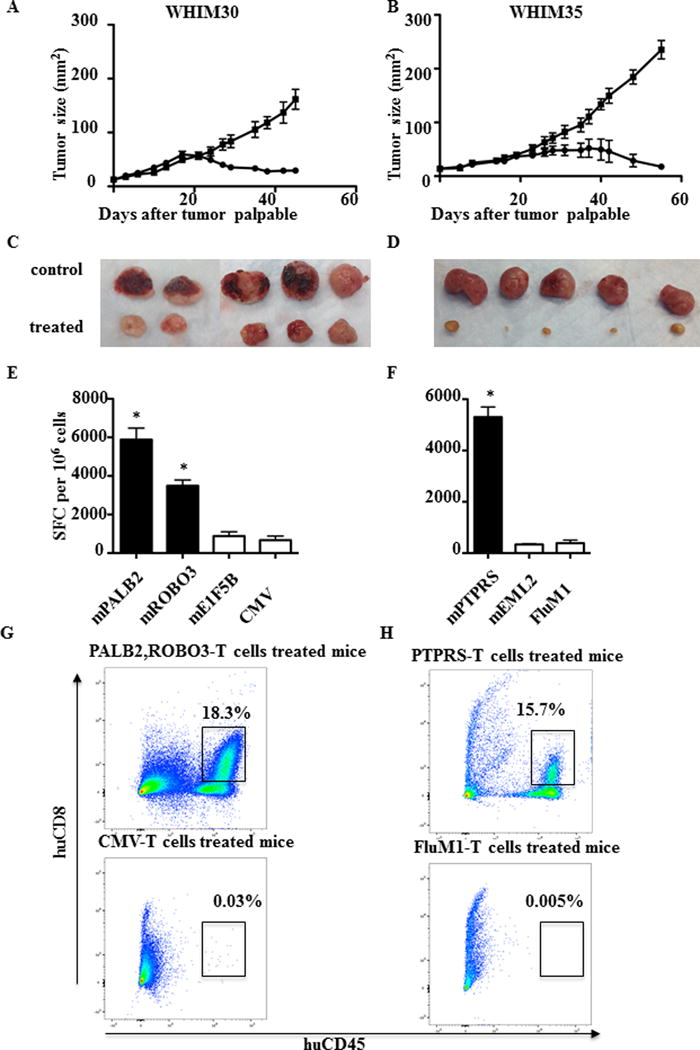
PDXs were established by injection of 1 × 106 tumor cells subcutaneously into NSG mice. 5–10 × 106 neoantigen-stimulated T cells and control viral-antigen stimulated T cells were adoptively transferred into tumor-bearing mice after the tumor became palpable. T cells were transferred at days 0, 7, and 14. Tumor size was measured every two days. (A) WHIM30 tumor growth following treatment with PBMC stimulated with mutant PALB2/ROBO3 (black solid circle) or CMV peptides (black solid square). (B) WHIM35 tumor growth following treatment with PBMC stimulated with mutant PTPRS (black solid circle) or FluM1 peptides (black solid square). (A) and (B) Data are presented as tumor size (mm2) ± s.e.m. of 5 mice per group (*P < 0.05). (C) WHIM30 tumor size (52 days after tumor challenge) following treatment with PBMC stimulated with mutant PALB2/ROBO3 or CMV peptides. (D) WHIM35 tumor size (62 days after tumor challenge) following treatment with PBMC stimulated with mutant PTPRS or CMV peptides. (E, G) TILs were isolated from WHIM30 tumors following treatment with PBMC stimulated with mutant PALB2/ROBO3 (black) or negative control mutant E1F5B and CMV (white) peptides. CD8+ T-cell IFNγ ELISPOT assay and flow cytometry were performed with experimental and control peptides as indicated. (F, H) TILs were isolated from WHIM30 tumors following treatment with PBMC stimulated with mutant PTPRS (black) or negative control mutant EML2 and FluM1 peptides (white). CD8+ T-cell mIFNγ ELISPOT assay and flow cytometry were performed with experimental and control peptides as indicated. (E) and (F) Data shown are mean ± s.e.m (n = 3 wells per peptide in ELISpot assay). Data shown are representative of three independent experiments. Samples were compared using an unpaired, two-tailed Student t test (P < 0.05).
