Abstract
The aim of the present study was to observe the dynamic changes of the growth arrest and DNA damage-inducible 153 (GADD153) gene and caspase-12 in the brain tissue of rats with cerebral ischemia-reperfusion injury (CIRI) and the impact of curcumin pretreatment. A total of 60 rats were randomly divided into the normal group (N), the sham operation group (S), the dimethyl sulfoxide control group (D) and the curcumin treatment group (C). For group D and C, 12 (T1), 24 (T2) and 72 h (T3) of reperfusion were performed after 2 h ischemia. The expression levels of GADD153 and caspase-12 in the brain tissue were detected and compared among the groups by immunohistochemistry, immunofluorescence double staining and western blotting. The expression levels of GADD153 and caspase-12 were increased at T1compared with groups N and S, and the expression of caspase-12 peaked at T2 in group D, while GADD153 was increased until T3 in group D. Compared with group D, the expression levels of GADD153 and caspase-12 in group C at T2 and T3 were significantly decreased (P<0.05). Endoplasmic reticulum stress is involved in the pathological process of CIRI. Curcumin may decrease the expression levels of the above two factors, thus exhibiting protective effects against CIRI in rats.
Keywords: ischemia-reperfusion, brain, growth arrest and DNA damage-inducible 153 gene, caspase-12, endoplasmic reticulum stress, curcumin
Introduction
The endoplasmic reticulum (ER) is the main site for the synthesis, processing and transport of the majority of intracellular, cell surface and extracellular proteins. ER functions may be disturbed by different factors, such as the accumulation of unfolded proteins and changes in Ca2+ homeostasis (1,2), which result in ER stress (ERS). The accumulation of misfolded proteins within the ERS triggers a pro-survival adaptation, the unfolded protein response (UPR) (3). Protein aggregates and Ca2+ homeostasis have been reported to accumulate following transient cerebral ischemia (4–6). Furthermore, aggregate formation coincided with the time course of cell death (4). Cumulative evidence suggests that signaling markers of the UPR are activated following cerebral ischemia (7–10). ERS is the key link of cerebral ischemia-reperfusion injury (CIRI) (8).
Curcumin is a low molecular weight polyphenol isolated from Curcuma longa, and has attracted increasing interest due to its roles against oxidative stress, inhibiting the release of inflammatory cytokines, anti-apoptosis and anti-tumor agents (11,12). Curcumin has neuroprotective effects against neurological diseases, such as cerebral ischemia, Parkinson's disease and Alzheimer's disease (13–17). Therefore, the present study investigated the dynamic changes of ERS apoptosis-related proteins, growth arrest and DNA damage-inducible 153 (GADD153) gene and caspase-12 in the CIRI process and the impact of curcumin pretreatment on the expression levels of GADD153 and caspase-12. The aim of the present study was to explore the effects of curcumin on ERS and to determine its neuroprotective effects.
Materials and methods
Animals and grouping
A total of 76healthy adult male Wistar rats (weighing 240–270 g, 7–8 weeks old) were provided by the Animal Experimental Center, School of Medicine, Shandong University (Jinan, China; certificate no. Ludongzhi 20001003). The rats were housed in a temperature (20±2°C) and humidity-controlled (50–60%) facility with a 12 h light/dark cycle and access to food and water ad libitum. First, 6 rats were randomly selected and divided into the sham operation group (group S) and the normal group (group N; n=3 per group). The remaining rats were used to create a CIRI model using the Longa method (18), and grouped into the dimethyl sulfoxide (DMSO) control group (group D) and the curcumin treatment group (group C; n=27 per group). For the rats in groups D and C, 12 (T1), 24 (T2) and 72 h (T3) of reperfusion were performed after 2-h ischemia (n=9 in each subgroup). The present study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (19). The animal use protocol was reviewed and approved by the Institutional Animal Care and Use Committee of Shandong Provincial Hospital (Jinan, China).
Establishment of a CIRI model
The rats in groups C and D were used to create a focal middle CIRI model using the modified Longa method (18). Each rat was anesthetized by intraperitoneally injecting 10% chloral hydrate (300 mg/kg; Shanghai Chemical Reagent Co., Ltd., Shanghai, China). After ligation of the distal end of the external carotid artery, one small incision was cut on the external carotid artery and one fishing line (0.25 mm in diameter, with the tip coated in silica gel) was inserted into the internal carotid artery for ~20 mm, and ligated. A total of 2 h later, the line bolt was gently pulled out by 15 mm as so to re-supply the blood flow to prepare the reperfusion model. In group S, the line was inserted~10 mm into the external carotid artery, and the rest of the procedure was the same as for groups C and D. Group N received no treatment.
The Longa nerve function score was used to evaluate the success of model preparation (18). Rats with a Longa score within 0 and 4 points were discarded, and the rats in which model preparation was successful (mean success rate=71%) were randomly selected for the supplement. Rats in group C were intraperitoneally injected with curcumin solution (150 mg/kg; concentration, 7.5 mg/ml, dissolved in DMSO; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) 2 h prior to modeling. Rats in group D were intraperitoneally injected with an equal volume of 10% DMSO solution (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) 2 h prior to modeling as the solvent control.
Reperfusion and sampling
The 54 rats modeled successfully were intraperitoneally injected with 10% chloral hydrate (400 mg/kg) and left atrial appendage perfusion was performed with 0.9% NaCl at T1, T2 and T3. At 2 h post-surgery, group S were anesthetized and perfused. Meanwhile, group N was anesthetized and perfused immediately. The brain tissue from the left cerebral hemisphere to the pituitary stalk was sampled, quickly frozen in liquid nitrogen, and stored at −70°C for the immunoblotting test. For the immunohistochemical staining and immunofluorescence double staining test, rats were perfused with 0.9% NaCl and paraformaldehyde (4%), respectively, and the whole brain tissue was fixed with 4% paraformaldehyde at 4°C for 24 h. The brain tissue from the left cerebral hemisphere to the pituitary stalk was subsequently sampled.
Immunohistochemistry
Conventional paraffin embedding was performed on the brain tissue and slices of 2–3 µm in thickness were created. Immunohistochemical staining was performed using a SABC kit (cat. no. SA1022; Wuhan Boster Biological Technology, Ltd., Wuhan, China), according to the manufacturer's instructions. The positive cells were characterized using an inverted microscope to observe brown stained cells in the cytoplasm and/or nucleus. A total of 10 random visual fields (magnification, ×40) of each of five slices of the cortex and corpus striatum in each rat were observed to calculate the rate of positive cells: Positive cells=(positive cells/total counted cells) ×100%. The mean number of positive cells in each group was then calculated: Mean number of positive cells in each group=total number of positive cells in each group/the number of the rats in each group. The standard deviation was then calculated.
Immunofluorescence double staining
The brain tissue was precipitated in 30% sucrose solution, sliced (~40-µm thick), stored at −20°C and the immunofluorescence double staining of GADD153 and caspase-12 was prepared by the section-bleaching method (20). Following blocking using 10% donkey serum (Guangzhou Ruite Biotechnology Co., Ltd., Guangzhou, China) at room temperature for 30 min, the slices were rinsed 3 times for 3 min with PBS and incubated with rabbit polyclonal GADD153 antibody (cat. no. sc-575) and goat polyclonal caspase-12 antibody (cat. no. sc-12395; both 1:150; both Santa Cruz Biotechnology, Inc.) overnight at 4°C. Subsequently, the slices were rinsed 5 times for 5 min with PBS and incubated for 1 h in darkness with donkey anti-rabbit immunoglobulin (Ig)G-Dylight 549 (cat. no. 711-505-152;Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA; 1:200) and donkey anti-goat IgG-Dylight 488 (cat. no. 705-485-003;, Jackson ImmunoResearch Laboratories, Inc.; 1:200) at 37°C.Observation and photography was performed using an immunofluorescence microscope. The GADD153 positive cells exhibited red fluorescence, and the caspase-12 positive cells exhibited green fluorescence, so the double-labeled positive cells exhibited yellow fluorescence. A total of 10 random visual fields (magnification, ×40) of each of five slices of the cortex and corpus striatum in each rat were observed to calculate the rate of positive cells and the average was calculated using the same method.
Western blotting
The left frontal and parietal lobes and the striatum were frozen in liquid nitrogen and stored at −70°C for western blotting. The tissue samples were homogenized in a lysis buffer [0.1 mol/l NaCl, 0.01 M Tris-HCl (pH 7.5), 1 mM EDTA and 1 µg/ml aprotinin], and then the homogenates were centrifuged at 12,000 × g for 5 min at 4°C. A Bradford assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used to determine the protein concentration, and western blotting was performed with a 5% acrylamide stacking gel and a 14% acrylamide resolving gel, with 80 µg protein per lane. Proteins were electrotransferred onto nitrocellulose membranes. Nonspecific protein binding to the nitrocellulose membrane was reduced by blocking the membranes with blocking buffer (5% nonfat dry milk, 2.7 mM KCl, 137 mM NaCl, 8 mM NaHPO4, 1.4 mM KPO4 and 0.1 % Tween 20) for 1 h at 37°C. Membranes were subsequently incubated with anti-β-actin (cat. no. RDP-105-025, 1:500; Chemicon; Merck KGaA), antiGADD153 (cat. no. SC-575; 1:500; Santa Cruz Biotechnology, Inc.) and anti-caspase-12 (cat. no. SC-12395; 1:500; Santa Cruz Biotechnology, Inc.) overnight at 4°C. Membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary anti-mouse IgG, (cat. no. ZDR-5307), HRP-anti-rabbit IgG (cat. no. ZDR-5306) and HRP-anti-goat IgG (cat. no. ZDR-5308; all 1:1,000; ZSGB-BIO, Beijing, China) for 1 h at 37°C. Bands were visualized using DAB reagent at room temperature for 2 min). The membrane was scanned with an imaging densitometer (SigmaScan program 4; Systat Software, Inc., San Jose, CA, USA), and the optical density was quantified.
Statistical analysis
SPSS v. 13.0 (SPSS, Inc., Chicago, IL, USA) was used for the statistical analysis. All results are expressed as the mean ± standard deviation. The individual groups were tested for differences using one-way analysis of variance with repeated measures, followed by Fisher's least significant difference test. P<0.05 was considered to indicate a statistically significant difference.
Results
Success rate of rat modeling
Among the 70 rats subjected to the modeling, 54 rats were successfully modeled, with a success rate of 71% (data not shown). In total, 5 rats lost consciousness (3 due to large area cerebral infarction, 1 due to subarachnoid hemorrhage, 1 due to anesthesia) and 6 rats succumbed in modeling (1 due to anesthesia death, 2 due to subarachnoid hemorrhage, 2 due to large area cerebral infarction, 1 due to pulmonary edema and hemorrhage, 1 due to unknown causes), and these 11 rats inhaled overdoses of isoflurane via chamber inhalation and mortality was confirmed. Of these, 5 rats exhibited no symptoms of neurological deficits following surgery. Prior to immunohistochemistry, double staining and western blotting, the 54 rats were sacrificed by chamber inhalation of overdoses of isoflurane and mortality was confirmed.
Immunohistochemical staining
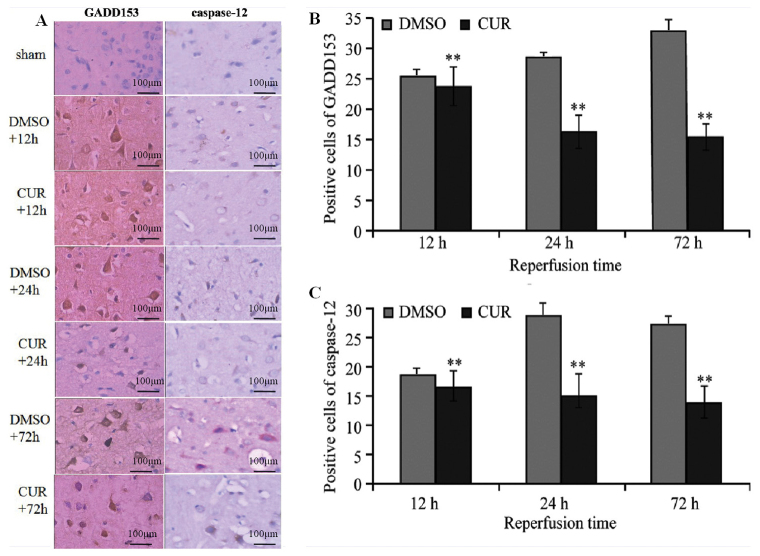
GADD153 and caspase-12 were not expressed in groups N and S (data not shown), while tan granular deposits were observed in the neurons, as well as the nuclei and cytoplasm of glial cells in group C and D at T1, T2 and T3 (Fig. 1A). Group D exhibited significantly more GADD153- and caspase-12-positive cells at T1 compared with group C (P<0.01). The number of GADD153-positive cells in group D increased at T2 and reached a peak at T3, whereas the number of caspase-12-positive cells in group D peaked at T2. Compared with group D, the expression of GADD153 and caspase-12 in group C at T2 and T3 were significantly decreased (P<0.01; Fig. 1B and C).
Figure 1.
Immunohistochemical staining of GADD153 and caspase-12. (A) Immunohistochemical staining results of GADD153 and caspase-12 in the ischemic brain tissue of rats (scale bar, 100 µm). Comparison of (B) GADD153- and (C) caspase-12-positive cells in the ischemic brain area between the CUR and DMSO groups. **P<0.01 vs. DMSO group. GADD153, growth arrest and DNA damage-inducible 153; CUR, curcumin; DMSO, dimethyl sulfoxide.
Immunofluorescence double staining
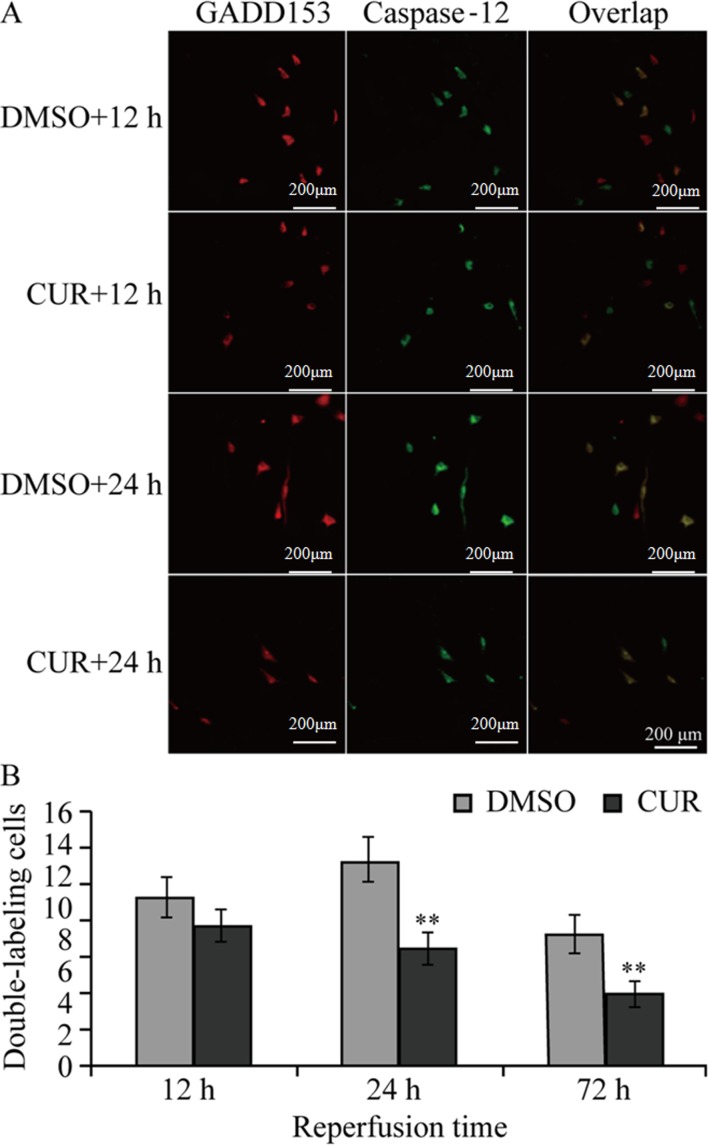
GADD153 and caspase-12 were not expressed in groups N and S (data not shown), while group C and D exhibited GADD153- and caspase-12-positive staining (single or double staining) at the three time points (Fig. 2A).
Figure 2.
Immunofluorescence double staining of GADD153 and caspase-12. (A) Immunofluorescence double staining results of GADD153 and caspase-12 in the ischemic brain area of rats in the DMSO and CUR groups. GADD153-positive cells are stained red, caspase-12-positive cells are stained green and dual-stained cells are colored yellow (scale bar, 200 µm). (B) Comparison of GADD153- and caspase-12-positive cells (dual-stained) in the ischemic brain area between the DMSO and CUR groups. **P<0.01 vs. DMSO group. GADD153, growth arrest and DNA damage-inducible 153; CUR, curcumin; DMSO, dimethyl sulfoxide.
Group D exhibited single-stained GADD153- and caspase-12-positive cells at T1, as well as small amounts of dual-stained GADD153- and caspase-12-positive cells, which peaked at T2. The number of single-stained caspase-12-positive cells was reduced while that of single-stained GADD153-positive cells was high, and the total number of dual-stained cells was reduced in Group D at T3 (72 h). Compared with group D, the numbers of dual-stained GADD153 and caspase-12 positive cells in group C at T2 and T3 were significantly decreased (P<0.01; Fig. 2B).
Western blot
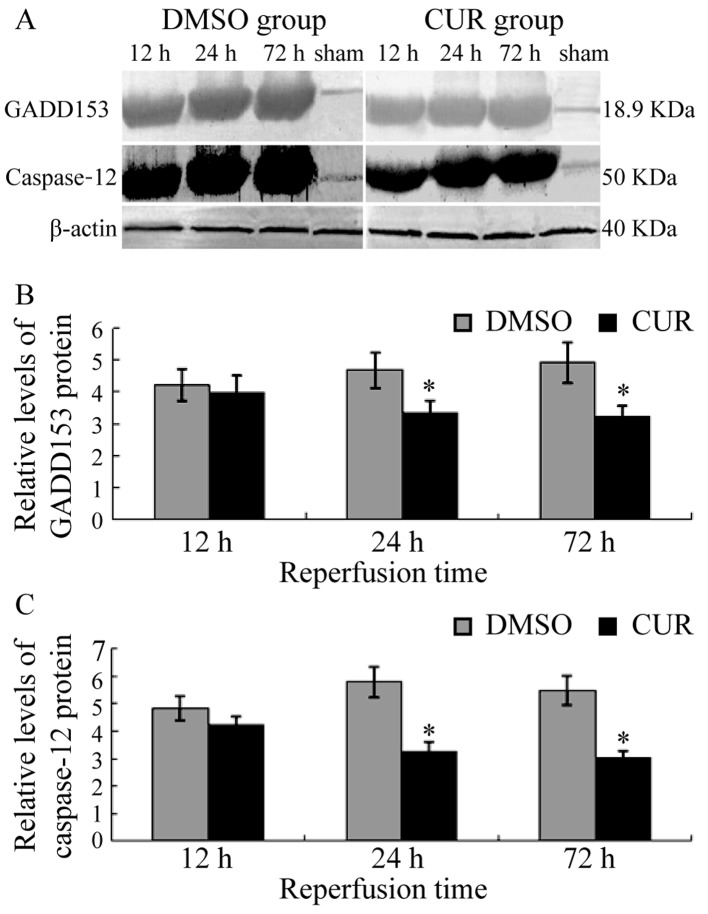
GADD153 and caspase-12 were not expressed in the brain tissue of groups N and S (data not shown). Group D exhibited the expression of GADD153 at T1, which was upregulated gradually and exhibited a high level until T3. Caspase-12 was expressed in group D at T1, reached a peak at T2 and slightly decreased at T3. Compared with group D, the expression levels of GADD153 and caspase-12 in group C were significantly decreased at T2 and T3 (P<0.05; Fig. 3).
Figure 3.
Western blot analysis results of GADD153 and caspase-12. (A) Western blotting results of GADD153 and caspase-12 in the ischemic brain area of rats in the DMSO and CUR groups. Comparison of (B) GADD153 and (C) caspase-12 protein expression levels between the DMSO and CUR groups by western blotting. *P<0.05 vs. DMSO group. GADD153, growth arrest and DNA damage-inducible 153; CUR, curcumin; DMSO, dimethyl sulfoxide.
Discussion
Studies have suggested that multiple causes of ERS occur in neurons following CIRI, including depletion of ER Ca2+, aggregation of proteins, decreased protein degradation and accumulation of lipid peroxidation products in ER and Golgi structures (21). The evidence summarized highlights early ER signaling events triggered by ischemia contribute to the restoration of homeostasis (22,23). However, prolonged ERS may trigger pro-apoptotic processes and lead to apoptosis.
ERS-mediated apoptosis occurs via a variety of ways, among which GADD153 and caspase-12 are two important pathways (24). GADD153 is an ERS-inducing protein that participates in ERS-related apoptosis (25). GADD153 is an important intermediate signaling molecule associated with ERS and apoptosis, and it may promote apoptosis by various pathways, such as affecting intracellular Ca2+ metabolism and downregulating B-cell lymphoma 2 (26–28). Caspase-12 belongs to the conserved caspase family, which is another major factor in regulating ERS-mediated apoptosis. Caspase-12 is located in the cytoplasm of the ER and may be activated by excessive ERS, including the damage of ER Ca2+ balance or excessive protein deposition in the ER; however, non-ERS-mediated apoptosis does not involve activation of caspase-12 (29). Therefore, caspase-12 is inherent in ERS-mediated apoptosis. When ERS occurs, caspase-12 is released from the ER, thus further activating the caspase cascade and leading to apoptosis (29).
Curcumin is a polyphenolic compound and the active ingredient of C. longa (30). Its chemical structure is C21H20O6 (molecular weight, 368.37 g/mol), including the enol type and keto type. Numerous studies have demonstrated that curcumin has anti-apoptotic effects. A study by Jutooru et al (31) indicated that curcumin may have an anti-apoptotic role through reducing nuclear factor (NF)-κB activity and the expression of its regulatory gene. A study by Chhunchha et al (32) demonstrated that curcumin may increase the expression of peroxiredoxin 6 (prdx6), thereby reducing hypoxia-induced hippocampal ERS and the oxidative stress response, and reducing the apoptosis of hippocampal cells, eliciting neuroprotective effects.
The results of the present study demonstrated that GADD153 and caspase-12 were expressed in the neurons and glial cells of rats with CIRI, which also indicated dynamic changes with time. At 12 h of reperfusion, the expression levels of GADD153 and caspase-12 were increased, caspase-12 peaked at 24 h, while GADD153 peaked at the 72 h. The results of immunofluorescence staining demonstrated that GADD153 was increased markedly compared with caspase-12 at T1, while both were markedly increased at T2 (more positive dual-stained cells); however, at T3, GADD153 was decreased while caspase-12 increased (positive dual-stained cells were decreased), suggesting that the activation of GADD153 occupies the main status in early perfusion stages, which activates various apoptosis factors. As CIRI worsens, caspase-12 is largely activated. GADD153 and caspase-12 then act together to promote the progression of ERS and aggravate apoptosis. The present study further demonstrated that ERS is involved in the pathological process of CIRI.
The expression levels of GADD153 and caspase-12 in group C at T1 were not significantly different to those in group D; however, they were significantly decreased at T2 and T3, suggesting that curcumin may reduce the expression levels of ERS apoptosis-related proteins GADD153 and caspase-12, thus reducing ERS and having neuroprotective effects. Previous studies have demonstrated that curcumin may reduce blood-brain barrier damage in rats with focal cerebral ischemia and have neuroprotective effects (13). A study by Jiang et al (14) reported, based on the SH-SY5Y cell line of Parkinson's disease, that curcumin may induce macro-autophagy, thus exhibiting neuroprotective effects. Curcumin may also protect amyloid β-induced mitochondrial and synaptic toxicities in Alzheimer's disease (15). However, the inhibitory mechanism of curcumin on ERS is not clear, and this may be realized through inhibiting the ERS cascade by decreasing the activity of NF-κB, increasing the expression of prdx6 or activating the Sirtuin type 1 pathway (26–34). Therefore, further research is required for clarification.
The results of the present study suggest that ERS is associated with the pathological progression of CIRI. Curcumin may decrease the expression levels of the above two factors, thus exhibiting protective effects against CIRI in rats.
References
- 1.Verkhratsky A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol Rev. 2005;85:201–279. doi: 10.1152/physrev.00004.2004. [DOI] [PubMed] [Google Scholar]
- 2.Boyce M, Yuan J. Cellular response to endoplasmic reticulum stress: A matter of life or death. Cell Death Differ. 2006;13:363–373. doi: 10.1038/sj.cdd.4401817. [DOI] [PubMed] [Google Scholar]
- 3.Hetz C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 4.Hu BR, Martone ME, Jones YZ, Liu CL. Protein aggregation after transient cerebral ischemia. J Neurosci. 2000;20:3191–3199. doi: 10.1523/JNEUROSCI.20-09-03191.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ge P, Luo Y, Liu CL, Hu B. Protein aggregation and proteasome dysfunction after brain ischemia. Stroke. 2007;38:3230–3236. doi: 10.1161/STROKEAHA.107.487108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohno K, Higuchi T, Ohta S, Kohno K, Kumon Y, Sakaki S. Neuroprotective nitric oxide synthase inhibitor reduces intracellular calcium accumulation following transient global ischemia in the gerbil. Neurosci Lett. 1997;224:17–20. doi: 10.1016/S0304-3940(97)13459-0. [DOI] [PubMed] [Google Scholar]
- 7.Roberts GG, Di Loreto MJ, Marshall M, Wang J, DeGracia DJ. Hippocampal cellular stress responses after global brain ischemia and reperfusion. Antioxid Redox Signal. 2007;9:2265–2275. doi: 10.1089/ars.2007.1786. [DOI] [PubMed] [Google Scholar]
- 8.Nakka VP, Gusain A, Raghubir R. Endoplasmic reticulum stress plays critical role in brain damage after cerebral ischemia/reperfusion in rats. Neurotox Res. 2010;17:189–202. doi: 10.1007/s12640-009-9110-5. [DOI] [PubMed] [Google Scholar]
- 9.Shibata M, Hattori H, Sasaki T, Gotoh J, Hamada J, Fukuuchi Y. Activation of caspase-12 by endoplasmic reticulum stress induced by transient middle cerebral artery occlusion in mice. Neuroscience. 2003;118:491–499. doi: 10.1016/S0306-4522(02)00910-7. [DOI] [PubMed] [Google Scholar]
- 10.Tajiri S, Oyadomari S, Yano S, Morioka M, Gotoh T, Hamada JI, Ushio Y, Mori M. Ischemic-induced neuronal cell death is mediated by the endoplasmic reticulum stress pathway involving CHOP. Cell Death Differ. 2004;11:403–415. doi: 10.1038/sj.cdd.4401365. [DOI] [PubMed] [Google Scholar]
- 11.Zhou H, Beevers CS, Huang S. The targets of curcumin. Curr Drug Targets. 2011;12:332–347. doi: 10.2174/138945011794815356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Onder A, Kapan M, Gümüş M, Yüksel H, Böyük A, Alp H, Başarili MK, Firat U. The protective effects of curcumin on intestine and remote organs against mesenteric ischemia/reperfusion injury. Turk J Gastroenterol. 2012;23:141–147. doi: 10.4318/tjg.2012.0446. [DOI] [PubMed] [Google Scholar]
- 13.Jiang J, Wang W, Sun YJ, Hu M, Li F, Zhu DY. Neuroprotective effect of curcumin on focal cerebral ischemic rats by preventing blood-brain barrier damage. Eur J Pharmacol. 2007;561:54–62. doi: 10.1016/j.ejphar.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 14.Jiang TF, Zhang YJ, Zhou HY, Wang HM, Tian LP, Liu J, Ding JQ, Chen SD. Curcumin ameliorates the neurodegenerative pathology in A53T α-synuclein cell model of Parkinson's disease through the downregulation of mTOR/p70S6K signaling and the recovery of macroautophagy. J Neuroimmune Pharmacol. 2013;8:356–369. doi: 10.1007/s11481-012-9431-7. [DOI] [PubMed] [Google Scholar]
- 15.Reddy PH, Manczak M, Yin X, Grady MC, Mitchell A, Kandimalla R, Kuruva CS. Protective effects of a natural product, curcumin, against amyloid β induced mitochondrial and synaptic toxicities in Alzheimer's disease. J Investig Med. 2016;64:1220–1234. doi: 10.1136/jim-2016-000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng HL, Dang HZ, Fan H, Chen XP, Rao YX, Ren Y, Yang JD, Shi J, Wang PW, Tian JZ. Curcumin ameliorates insulin signaling pathway in brain of Alzheimer's disease transgenic mice. Int J Immunopathol Pharmacol. 2016;29:734–741. doi: 10.1177/0394632016659494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pluta R, Bogucka-Kocka A, Ułamek-Kozioł M, Furmaga-Jabłońska W, Januszewski S, Brzozowska J, Jabłoński M, Kocki J. Neurogenesis and neuroprotection in postischemic brain neurodegeneration with Alzheimer phenotype: Is there a role for curcumin? Folia Neuropathol. 2015;53:89–99. doi: 10.5114/fn.2015.52405. [DOI] [PubMed] [Google Scholar]
- 18.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.STR.20.1.84. [DOI] [PubMed] [Google Scholar]
- 19.Guide for the Care & Use of Laboratory Animals. 8th. Washington (DC): National Academies Press (US); 2011. National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the care and use of laboratory animals. [Google Scholar]
- 20.Yao H, Gao J, Feng YB, Pang ZY, Chi ZF. 2R, 4R-APDC decreases cell proliferation in the dentate gyrus of adult rats: The effect of 2R, 4R-APDC on cell proliferation. Neuroreport. 2007;18:1459–1462. doi: 10.1097/WNR.0b013e3282ef773e. [DOI] [PubMed] [Google Scholar]
- 21.DeGracia DJ, Montie HL. Cerebral ischemia and the unfolded protein response. J Neurochem. 2004;91:1–8. doi: 10.1111/j.1471-4159.2004.02703.x. [DOI] [PubMed] [Google Scholar]
- 22.Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, Yuan J. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- 23.Lee DY, Lee KS, Lee HJ, Kim DH, Noh YH, Yu K, Jung HY, Lee SH, Lee JY, Youn YC, et al. Activation of PERK signaling attenuates Abeta-mediated ER stress. PLoS One. 2010;5:e10489. doi: 10.1371/journal.pone.0010489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamkanfi M, Kalai M, Vandenabeele P. Caspase-12: An overview. Cell Death Differ. 2004;11:365–368. doi: 10.1038/sj.cdd.4401364. [DOI] [PubMed] [Google Scholar]
- 25.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 26.Li G, Mongillo M, Chin KT, Harding H, Ron D, Marks AR, Tabas I. Role of ERO1-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J Cell Biol. 2009;186:783–792. doi: 10.1083/jcb.200904060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Timmins JM, Ozcan L, Seimon TA, Li G, Malagelada C, Backs J, Backs T, Bassel-Duby R, Olson EN, Anderson ME, Tabas I. Calcium/calmodulin-dependent protein kinase II links endoplasmic reticulum stress with Fas and mitochondrial apoptosis pathways. J Clin Invest. 2009;119:2925–2941. doi: 10.1172/JCI38857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl-2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferri KF, Kroemer G. Organelle-specific initiation of cell death pathways. Nat Cell Biol. 2001;3:E255–E263. doi: 10.1038/ncb1101-e255. [DOI] [PubMed] [Google Scholar]
- 30.Maheshwari RK, Singh AK, Gaddipati J, Srimal RC. Multiple biological activities of curcumin: A short review. Life Sci. 2006;78:2081–2087. doi: 10.1016/j.lfs.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Jutooru I, Chadalapaka G, Lei P, Safe S. Inhibition of NFkappaB and pancreatic cancer cell and tumor growth by curcumin is dependent on specificity protein downregulation. J Biol Chem. 2010;285:25332–25344. doi: 10.1074/jbc.M109.095240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chhunchha B, Fatma N, Kubo E, Rai P, Singh SP, Singh DP. Curcumin abates hypoxia-induced oxidative stress based-ER stress-mediated cell death in mouse hippocampal cells (HT22) by controlling Prdx6 and NF-κB regulation. Am J Physiol Cell Physiol. 2013;304:C636–C655. doi: 10.1152/ajpcell.00345.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun Q, Jia N, Wang W, Jin H, Xu J, Hu H. Activation of SIRT1 by curcumin blocks the neurotoxicity of amyloid-β25–35 in rat cortical neurons. Biochem Biophys Res Commun. 2014;448:89–94. doi: 10.1016/j.bbrc.2014.04.066. [DOI] [PubMed] [Google Scholar]
- 34.Wang L, Zhang B, Huang F, Liu B, Xie Y. Curcumin inhibits lipolysis via suppression of ER stress in adipose tissue and prevents hepatic insulin resistance. J Lipid Res. 2016;57:1243–1255. doi: 10.1194/jlr.M067397. [DOI] [PMC free article] [PubMed] [Google Scholar]