Table 2.
Buchwald–Hartwig etherifications of bromoalcohol 15.
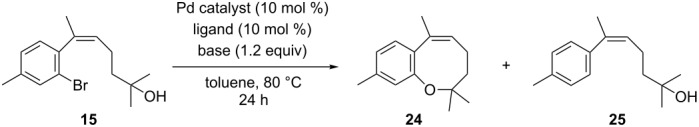 | |||||
| Entry | Pd catalyst | Ligand | Base | Yield(%) | |
| 24 | 25 | ||||
| 1 | Pd(OAc)2 | tol-BINAP | K2CO3 | 0 | 0a |
| 2 | Pd(TFA)2 | tol-BINAP | Cs2CO3 | 0 | 21 |
| 3 | Pd(OAc)2 | dppf | NaH | 0 | 45b |
| 4 | Pd(dba)2 | Q-Phos | NaOt-Bu | 7 | 53 |
| 5 | Pd(dba)2 | Q-Phos | NaH | 0 | 40 |
| 6 | Pd2(dba)3 | Q-Phos | NaOt-Bu | 10 | 12 |
| 7c | Pd(dba)2 | Q-Phos | NaOt-Bu | 6 | 21d |
| 8 | Pd(dba)2 | Q-Phos | DBU | 0 | 0e |
a73% starting material recovered. bUsing dioxane as solvent gave a 32% yield of 25. cMicrowave heating, 80 °C, 18 h. d25% starting material recovered. e65% starting material recovered.
