Abstract
Objectives
To report the results of an intervention using the 4 Pillars™ Practice Transformation Program (4 Pillars™ Program) to increase adolescent vaccinations including human papillomavirus vaccine (HPV) and influenza vaccines, which remain underutilized in this population.
Study Design
Eleven pediatric and family medicine practices, previously control sites from a randomized controlled cluster trial, with ≥50 adolescent patients participated. The 4 Pillars™ Program was the foundation of the intervention. De-identified demographic, office visit and vaccination data were derived from electronic medical record extractions for patients whose date of birth was 4/1/1997 to 4/1/2004 (ages 11–17 years at baseline). Vaccination rates for HPV, influenza, tetanus-pertussis-diphtheria (Tdap) and meningococcal (MenACWY) vaccines were determined for all eligible patients pre- and post intervention (i.e., vaccination rates on 4/1/2015 and 4/30/2016).
Results
Among 9,473 patients ages 11–17 years at baseline (4/1/2015), mean pre-intervention vaccination rates for HPV initiation and completion, meningococcal, Tdap and influenza vaccines were below national levels. Rates increased significantly post intervention (P<0.001) for HPV initiation which increased 17.1 percentage points (PP) from 51.4%; HPV completion increased 14.8 PP from 30.7%, meningococcal vaccine uptake increased 16.6 PP from 79.1%, Tdap vaccine uptake increased 14.6 PP from 76.9%. Influenza vaccine uptake did not increase significantly (2.3 PP from 40.1%). In the regression using generalized estimating equations, odds of vaccination were higher for younger, non-white adolescents for all vaccines; being in a smaller practice decreased the odds of Tdap vaccination but increased the odds of influenza vaccination.
Conclusion
Clinically and statistically significant improvements in HPV series initiation and completion, and meningococcal and Tdap vaccinations were observed in primary care practices implementing the 4 Pillars™ Practice Transformation Program.
Keywords: adolescent vaccines, 4 Pillars, HPV vaccination, Tdap vaccination
INTRODUCTION
Four vaccines are currently recommended for adolescents ages 11–17 years - human papillomavirus (HPV), influenza vaccine, tetanus-pertussis-diphtheria (Tdap) vaccine and meningococcal conjugate vaccine (MenACWY) hereinafter referred to as meningococcal vaccine. Efforts to improve vaccination among adolescents have primarily focused provider and patient-based interventions for HPV and influenza vaccination and state mandates for meningococcal and Tdap vaccines. As a result, national adolescent vaccination rates are improving. However, the United States (U.S.) average HPV dose 1 (vaccine series initiation), dose 3 (series completion), and influenza vaccination rates are below recommended levels.1 Additionally, there is considerable variability in uptake of all four of these vaccines among the states. Thus, further efforts to increase vaccination are needed to protect adolescents.
Interventions to improve HPV uptake have taken many approaches, including practice-, provider-, and patient/parent-focused interventions and have met with varying success. A review of interventions to increase HPV vaccinations suggested that health care settings are a desirable environment for successful vaccination interventions.2 This conclusion is strengthened by the preponderance of evidence that links physician recommendation to uptake of HPV vaccine.3,4
The unique structure and culture of primary care practices has been shown to affect the acceptability, use and degree of success of a practice improvement program to increase adult immunizations,5 suggesting the need for a program that is adaptable to the unique settings and patient populations of diverse practices. The 4 Pillars™ Practice Transformation Program (4 Pillars™ Program) is a web-based quality improvement tool (www.4pillarstransformation.pitt.edu) developed to assist primary care providers with increasing adult and adolescent vaccinations. Its individualized program guidance and strategies allow practices to create an intervention that will likely work within their practice setting.
In a randomized controlled cluster trial, the 4 Pillars™ Program was shown to assist primary care practices with increasing Tdap, meningococcal, and HPV series initiation rates.6 Control sites from that study were subsequently offered the intervention. This study reports the findings from the intervention offered to those eleven primary care control practices in a pre-post design. This study took place before the HPV recommendation changed from a 3-dose series to a 2-dose series in 2016.7
METHODS
This study was approved by the Institutional Review Board of the University of Pittsburgh. Practices, not individual patients, were the unit of intervention because this intervention took place at the practice level and individual consent was not required. Practice level consent was implied by agreement of the practice leaders (lead physician, practice or office manager) to participate in the study.
Participating Practices
Primary care family medicine and pediatric practices from two practice-based research networks and a clinical network in the Pittsburgh metropolitan area were solicited in 2013 for participation in a randomized controlled cluster trial (RCCT). Practices were selected for solicitation based on preliminary estimates of patient volume and vaccination rates. Eligibility requirements included having an adolescent practice of at least 50 patients, estimated vaccination rates for at least one adolescent vaccine (HPV, Tdap, meningococcal, influenza) less than national goals (80%)1 and a willingness to make office changes to increase vaccination rates. All sites used a common electronic medical record (EMR), EpicCare. Twenty practices participated in the RCCT, the methods and results of which have been previously published.6 This pre-post study took place during 2015–2016, and reports on analysis of the 11 control sites that had waited a year to receive the intervention.
Intervention
The intervention was designed using Diffusion of Innovations theory,8 and included the 4 Pillars™ Program, provider education, and one-on-one coaching of a Champion for each site. The 4 Pillars™ Practice Transformation Program is founded on four key, evidence-based9,10 domains: Pillar 1 - Convenient vaccination services; Pillar 2 – Communication with patients about the importance of immunization and the availability of vaccines; Pillar 3 - Enhanced office systems to facilitate immunization; Pillar 4 - Motivation through an office Immunization Champion. Practices were expected to implement strategies from each of the 4 pillars, and were encouraged to use as many strategies as possible and appropriate for the practice to maximize their impact on vaccination rates. Strategies included using every opportunity to vaccinate, routinizing vaccination by using standing order protocols for nursing staff to vaccinate, sending reminders to patients to return for subsequent doses and office-wide recommendations to parents/patients to be vaccinated. The web-based 4 Pillars™ Program also included a practice transformation dashboard (PTD) that included an at-a-glance summary of the practice’s unique information and program status, such as a listing of the selected intervention strategies and a task list of incomplete intervention activities sorted by suggested due date.
The intervention was introduced in a three-stage process. A research liaison (KKM) visited each site to introduce the study and the 4 Pillars™ Program to key leaders of the practice such as the lead physician, office manager and/or head nurse. Those individuals then selected an Immunization Champion with whom the research liaison met at a second site visit to more fully introduce the intervention and the 4 Pillars™ Program and empower the Immunization Champion. At a third meeting, the Immunization Champion, along with the head nurse or office manager, presented the study to the staff and worked with them to develop practice-specific ideas for implementing strategies from each of the 4 pillars. With the assistance of the research liaison as needed, the Immunization Champion registered the practice on the website, entered chosen strategies and used the 4 Pillars™ Program to guide their efforts. Other roles for the Champion included receiving progress reports on the number of HPV and influenza vaccines given every two weeks, working to motivate the staff accordingly, and participating in biweekly telephone coaching with the research liaison.
Data collection
De-identified demographic, office visit and vaccination data were derived from EMR data extractions. A data base was created with only those patients who were 11–17 years (date of birth between 4/1/1997 and 4/1/2004), who were seen during the intervention period. For patients with ≥1 visit during the intervention period, vaccination status and age were derived from their most recent visit. Practice level vaccination rates for all eligible patients were determined at the beginning and the end of the intervention.
Statistical analyses
Descriptive analyses were performed for patient demographic characteristics including age group (11–13 years vs. 14–17 years), sex, race (white vs. non-white), and health insurance type (commercial vs. non-commercial) for each practice. The analytical period was 4/1/2015 through 4/30/2016. Proportions were reported for categorical variables and means and standard deviations were reported for continuous variables. At the time of the intervention, ACIP recommendations for HPV were to administer a 3-dose series beginning at day 0, and repeat 1–2 months later and at 6 months after dose 1. Thus, HPV vaccination rates were measured in two ways – series initiation and series completion. The primary outcome measures were the practice-level cumulative HPV series initiation and completion rates, cumulative meningococcal and Tdap vaccination rates and annual influenza vaccination rates, reported pre- and post-intervention. Student’s paired t-tests were performed to test for pre-post differences in influenza vaccination rates and cumulative HPV initiation, HPV completion, meningococcal and Tdap vaccination rates by clinics. One-way ANOVA was used to compare the changes in HPV vaccination rates between age groups.
To determine the factors associated with the odds of HPV series initiation, HPV series completion, Tdap, meningococcal, and influenza vaccination, respectively, generalized estimating equation (GEE) modeling, which accounts for the clustered nature of the data (i.e., patients are clustered within practices), was conducted using vaccination status for each vaccine as the binary outcome variable. Demographic characteristics (age, sex, race, health insurance type) and the practice characteristic of size <500 adolescent patients and >1000 adolescent patients were the independent variables. Demographic variables were included because they have been shown to be associated with varying vaccination rates. The size variable directly correlated with the type of practice, with the family medicine practices having fewer than 500 adolescent patients and the pediatric practices having 1000 or more adolescent patients. Statistical significance for two-sided tests was set at a type I error (alpha) equal to 0.05. All analytical procedures were performed using SAS® 9.4 (SAS Institute, Inc. Cary, NC, USA).
RESULTS
Across all eleven primary care practices, there were 9,473 patients ages 11–17 years at baseline (Table 1). One-half were females, nearly one-third were non-white and two-thirds were commercially insured. Table 2 shows pre- and postintervention vaccination rates and percentage point (PP) differences. Pre-intervention vaccination rates varied across sites from 35.6% to 64.8% for HPV initiation. The average increase across all sites was 17.1 PP (P<0.001) for HPV series initiation (range = 10.7–24.4 PP). Pre-intervention HPV series completion rate was lower (30.7%) ranging from 16.1% to 38.0% and increased 14.8 PP (P<0.001; range = 9.3–24.5 PP). When separated by age group (11–13 years and 14–17 years), the baseline HPV initiation rate was higher among older adolescents (65.2% older vs. 34% younger, respectively) but increases among younger adolescents (27.9 PP) surpassed those of older adolescents (9.2 PP; P<0.001). See supplemental table. HPV completion rate at baseline followed a similar pattern (11.1% for younger adolescents and 44.9% for older adolescents), but increases were similar between the two groups (17 PP for younger vs. 13.4 PP for older; P=0.19). While meningococcal (79.1%) and Tdap (76.9%) vaccination rates were moderately high pre-intervention, average PP increases were clinically and statistically significant at 16.6 PP (range = 9.4–21.4 PP) for meningococcal vaccine and 14.6 PP (range = 10.7–20.9 PP) for Tdap vaccine (P<0.001 for pre-post differences) (Table 2). Average influenza vaccination rates did not differ from pre- (40.6%) to post intervention (42.9%; 2.3 PP difference).
Table 1.
Baseline demographic characteristics and vaccination rates of participating practices (practices, N=11; patients ages 11–17, N=9,473)
| Vaccination rates | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Site | N | 11–13 years, % | Females, % | Non-white race, % | Commercial insurance, % | HPV initiation, % | HPV completion, % | Meningococcal, % | Tdap, % | Influenza, % |
| A | 158 | 38.6 | 60.8 | 3.2 | 47.5 | 48.8 | 38.0 | 83.5 | 83.6 | 17.0 |
| B | 160 | 27.5 | 49.4 | 6.3 | 63.8 | 44.6 | 26.6 | 80.9 | 81.9 | 21.8 |
| C | 165 | 28.5 | 43.0 | 10.3 | 64.2 | 64.8 | 29.2 | 75.5 | 75.8 | 23.6 |
| D | 242 | 31.4 | 50.1 | 10.7 | 59.9 | 65.8 | 34.8 | 78.6 | 79.0 | 17.7 |
| E | 258 | 38.0 | 53.9 | 45.4 | 15.9 | 51.8 | 24.4 | 70.1 | 69.4 | 46.5 |
| F | 422 | 35.1 | 55.5 | 88.4 | 32.0 | 41.7 | 32.2 | 63.9 | 60.7 | 22.7 |
| G | 1,120 | 40.4 | 50.1 | 1.2 | 82.2 | 35.6 | 20.0 | 76.8 | 79.3 | 40.2 |
| H | 1,368 | 42.0 | 48.7 | 30.5 | 78.4 | 38.1 | 27.2 | 69.0 | 70.9 | 51.6 |
| I | 1,400 | 44.1 | 48.7 | 11.6 | 89.9 | 54.2 | 33.1 | 84.5 | 84.7 | 46.4 |
| J | 2,066 | 48.6 | 49.4 | 88.1 | 25.5 | 47.4 | 30.9 | 79.9 | 80.6 | 39.2 |
| K | 2,114 | 41.7 | 48.3 | 2.0 | 68.7 | 35.1 | 16.1 | 74.3 | 74.7 | 40.9 |
|
| ||||||||||
| Total | 9,473 | 42.3 | 49.6 | 31.7 | 61.6 | 52.0 | 30.6 | 79.1 | 79.5 | 40.5 |
Note: HPV=human papillomavirus vaccine; Tdap=tetanus, diphtheria and pertussis vaccine
Table 2.
Vaccination rates and percentage point (PP) changes in vaccination post intervention
| HPV Initiation | HPV completion | Meningococcal | Tdap | Influenza | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | Pre, % | Post, % | PP* difference | Pre, % | Post, % | PP* difference | Pre, % | Post, % | PP* difference | Pre, % | Post, % | PP* difference | Pre, % | Post, % | PP* difference |
| A | 41.7 | 65.8 | 24.1 | 32.2 | 48.7 | 16.5 | 63.9 | 82.2 | 18.3 | 80.6 | 97.0 | 16.4 | 17.0 | 24.0 | 7.0 |
| B | 35.6 | 55.0 | 19.4 | 20.0 | 29.3 | 9.3 | 76.8 | 86.2 | 9.4 | 81.9 | 96.1 | 14.2 | 21.8 | 40.0 | 18.2 |
| C | 38.1 | 50.3 | 12.2 | 27.2 | 38.1 | 10.9 | 69.0 | 85.4 | 16.4 | 75.8 | 96.7 | 20.9 | 23.6 | 25.4 | 1.8 |
| D | 35.1 | 45.8 | 10.7 | 16.1 | 26.0 | 9.9 | 74.3 | 90.4 | 16.1 | 79.0 | 95.7 | 16.7 | 17.1 | 16.5 | −1.2 |
| E | 65.8 | 87.9 | 22.1 | 34.8 | 59.3 | 24.5 | 78.6 | 95.7 | 17.1 | 60.7 | 78.4 | 17.7 | 465 | 58.9 | 12.4 |
| F | 51.8 | 70.1 | 18.3 | 24.4 | 41.4 | 17.0 | 70.1 | 86.2 | 16.1 | 74.7 | 90.4 | 15.7 | 22.7 | 33.4 | 10.7 |
| G | 44.6 | 56.1 | 11.5 | 26.6 | 39.4 | 12.8 | 80.9 | 95.5 | 14.6 | 79.3 | 90.0 | 10.7 | 40.2 | 38.2 | −2.0 |
| H | 54.2 | 67.5 | 13.3 | 33.1 | 48.2 | 15.1 | 84.5 | 98.3 | 13.8 | 70.9 | 84.2 | 13.3 | 51.6 | 45.7 | −5.9 |
| I | 48.8 | 63.3 | 14.5 | 38.0 | 50.4 | 12.4 | 83.5 | 97.6 | 14.1 | 84.7 | 98.6 | 13.9 | 46.4 | 40.2 | −6.2 |
| J | 64.8 | 89.2 | 24.4 | 29.2 | 48.3 | 19.1 | 75.5 | 96.9 | 21.4 | 83.6 | 97.6 | 14.0 | 39.2 | 54.0 | 14.8 |
| K | 47.4 | 64.2 | 16.8 | 30.9 | 44.0 | 13.1 | 79.9 | 96.9 | 17.0 | 69.4 | 86.4 | 17.0 | 40.9 | 40.3 | −0.6 |
|
| |||||||||||||||
| Total | 51.4 | 69.1 | 17.1** | 30.7 | 45.5 | 14.8** | 79.1 | 95.7 | 16.6** | 76.9 | 91.5 | 14.6** | 40.6 | 42.9 | 2.3 |
Note: HPV=human papillomavirus vaccine; Tdap=tetanus, diphtheria and pertussis vaccine
PP=percentage point difference pre- to post intervention
P<0.001 for mean difference from pre- to post intervention by paired t-test
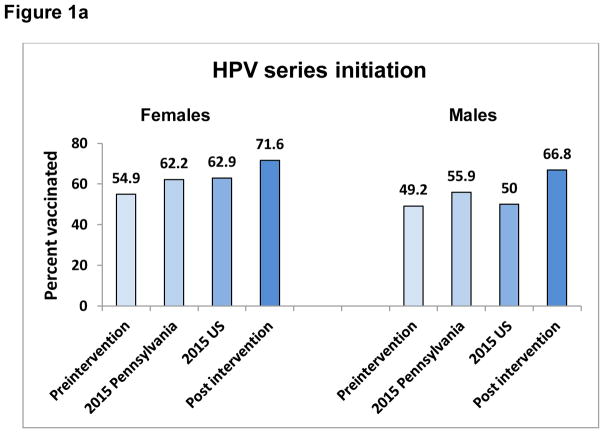
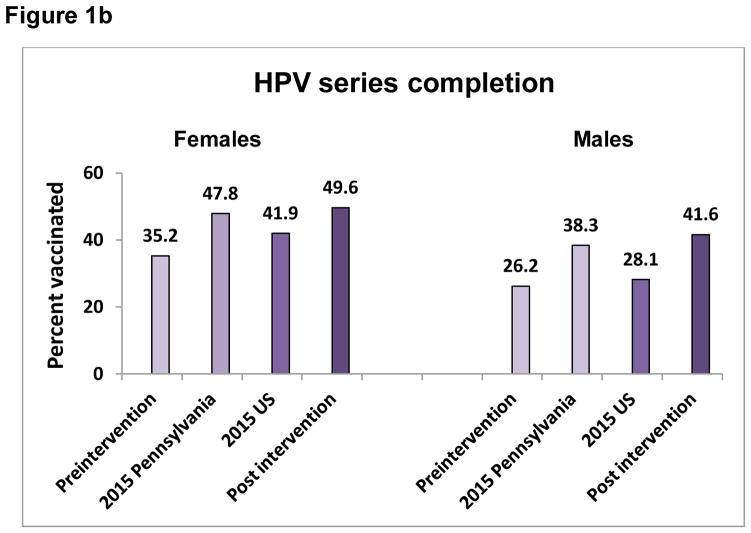
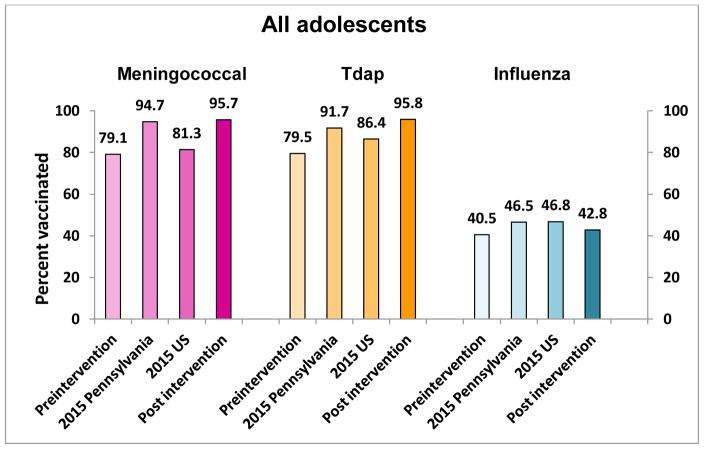
One site achieved the highest PP difference for 3 vaccines – HPV initiation, meningococcal and Tdap vaccines, one practice had the highest PP increase for HPV completion and another for influenza. Figures 1a, 1b, and 2 show the pre- and postintervention vaccination rates as well as the Pennsylvania and U.S. rates for the same time period, indicating comparable or higher post intervention rated than either Pennsylvania or the U.S.
Figures 1a and b. HPV series initiation (a) and completion (b) for Pennsylvania, United States, pre- and post intervention for adolescents 11–17 years, by sex.
Preintervention= rate on 4/1/2015; 2015 Pennsylvania= Pennsylvania 2015 reported rate; 2015 US=United States 2015 reported rate; Post intervention=rate on 4/30/2016
Figure 2. Meningococcal, Tdap and influenza vaccination rates for Pennsylvania, United States, pre- and post intervention among adolescents 11–17 years.
Preintervention= rate on 4/1/2015; 2015 Pennsylvania= Pennsylvania 2015 reported rate; 2015 US=United States 2015 reported rate; Post intervention=rate on 4/30/2016
In regression analyses, the variables significantly related to increased odds of HPV initiation and meningococcal vaccination were non-white race, age 11–13 years, and having public insurance; whereas, HPV series completion was related to non-white race and age 11–13 years (Table 3). Odds of Tdap and influenza vaccination was related to non-white race, age 11–13 years, and having public insurance. Being in a smaller practice (<500 adolescent patients) decreased the likelihood of receiving Tdap vaccine (OR=0.75; 95%CI=0.62–0.91) relative to being in a larger practice (>1000 adolescent patients), but increased the likelihood of influenza vaccination (OR=1.58; 95%CI=1.40–1.78).
Table 3.
Likelihood of vaccination by vaccine type from generalized estimating equation logistic regression
| HPV vaccine series initiation | |||
|---|---|---|---|
| Variable | Odds Ratio | 95% CI | P value |
| Non-white race, ref. = white | 1.43 | 1.27–1.62 | <0.001 |
| Female sex, ref. = male | 0.94 | 0.84–1.05 | 0.300 |
| Age 11–13 years, ref. = 14–17 years | 3.73 | 3.32–4.19 | <0.001 |
| Commercial insurance, ref. = public | 0.83 | 0.73–0.93 | 0.002 |
| Size of practice <500 adolescents, ref. = >1000 adolescents | 0.88 | 0.75–1.03 | 0.120 |
|
| |||
| HPV vaccine series completion | |||
| Variable | Odds Ratio | 95% CI | P value |
|
| |||
| Non-white race, ref. = white | 1.37 | 1.20–1.55 | <0.001 |
| Female sex, ref. = male | 0.92 | 0.82–1.03 | 0.149 |
| Age 11–13 years, ref. = 14–17 years | 1.31 | 1.17–1.47 | <0.001 |
| Commercial insurance, ref. = public | 1.00 | 0.89–1.14 | 0.893 |
| Size of practice <500 adolescents, ref. = >1000 adolescents | 0.94 | 0.80–1.10 | 0.462 |
|
| |||
| Meningococcal vaccine | |||
| Variable | Odds Ratio | 95% CI | P value |
|
| |||
| Non-white race, ref. = white | 1.11 | 1.01–1.23 | 0.039 |
| Female sex, ref. = male | 0.99 | 0.91–1.08 | 0.880 |
| Age 11–13 years, ref. = 14–17 years | 1.63 | 1.49–1.78 | <0.001 |
| Commercial insurance, ref. = public | 0.84 | 0.77–0.93 | <0.001 |
| Size of practice <500 adolescents, ref. = >1000 adolescents | 0.98 | 0.87–1.11 | 0.794 |
|
| |||
| Tdap vaccine | |||
| Variable | Odds Ratio | 95% CI | P value |
|
| |||
| Non-white race, ref. = white | 1.23 | 10.6–1.41 | 0.005 |
| Female sex, ref. = male | 1.08 | 0.96–1.22 | 0.209 |
| Age 11–13 years, ref. = 14–17 years | 38.04 | 30.55–47.37 | <0.001 |
| Commercial insurance, ref. = public | 0.75 | 0.65–0.86 | <0.001 |
| Size of practice <500 adolescents, ref. = >1000 adolescents | 0.75 | 0.62–0.91 | 0.003 |
|
| |||
| Influenza vaccine | |||
| Variable | Odds Ratio | 95% CI | P value |
|
| |||
| Non-white race, ref. = white | 1.32 | 1.20–1.45 | <0.001 |
| Female sex, ref. = male | 1.03 | 0.95–1.12 | 0.443 |
| Age 11–13 years, ref. = 14–17 years | 1.27 | 1.17–1.38 | <0.001 |
| Commercial insurance, ref. = public | 0.96 | 0.87–1.05 | 0.362 |
| Size of practice <500 adolescents, ref. = >1000 adolescents | 1.58 | 1.40–1.78 | <0.001 |
Note: HPV=human papillomavirus vaccine; Tdap=tetanus, diphtheria and pertussis vaccine
DISCUSSION
Using the 4 Pillars™ Practice Transformation Program and support from the research team, participating practices were able to improve overall adolescent vaccination rates including HPV series initiation, HPV series completion, meningococcal, Tdap and influenza. In most practices, vaccination increased by 10 percentage points or more. The exception was influenza vaccine for which changes were modest or decreased in some practices. These increases were higher than 10 PP increases in HPV initiation and 13 PP increases in HPV completion rates previously reported in the RCCT, in which these sites were the control group.11 This may be explained in part, by the longer follow-up period in this study, as well as the significant HPV initiation increases observed among these practices during the RCCT. Having already increased the number of adolescents receiving the first or second dose of HPV in effect, gave these practices a year-long period in which to complete the remaining doses of the 3-dose series. Interestingly, no single site had either the highest or lowest vaccination rates for all vaccines. One site was lowest for HPV initiation and completion, one site was lowest for meningococcal and Tdap vaccines and one site was lowest for influenza; whereas four different sites had the highest vaccination rates for the five vaccines.
Pre-intervention HPV series initiation and completion rates in participating practices were lower than both 2015 Pennsylvania and overall U.S. average rates for both females and males (Figure 1a and b). Post intervention, both HPV series initiation and completion rates were higher than the 2015 Pennsylvania and U.S. averages for females and males.12 In comparison with HPV initiation, completion of the HPV 3-dose series continues to be difficult, as evidenced by the lower rates (in comparison with HPV initiation) observed nationally, as well as in this and other studies.4,13 It remains to be seen whether completion of the newly recommended 2-dose schedule will be higher.
Previous studies have found that black adolescents and those of lower income are more likely to receive HPV dose 1 and less likely to receive HPV dose 3 than white and higher income adolescents.4 In this study, those racial differences were eliminated and in fact, the likelihood of HPV initiation was 43% higher and the likelihood of HPV completion was 37% higher for non-white adolescents than for their white counterparts.
Practices were encouraged to use strategies from the 4 Pillars™ Practice Transformation Program as appropriate to their setting. The sample size in this study precludes analyzing the effectiveness of specific strategies; however, in the RCCT related to this study, significant increases in vaccine uptake were related to adoption of >10 strategies.6 Combinations of provider- and patient-based strategies may address previously observed barriers to HPV vaccination among adolescents.14–18 Despite a Pennsylvania state mandate19 for children to receive meningococcal and Tdap vaccines at ages 11–12 years, pre-intervention vaccination rates among participating practices were below 2015 Pennsylvania and U.S. averages (Figure 2).12 Both meningococcal and Tdap average vaccination rates in participating practices increased to 95% post intervention, surpassing both Pennsylvania and U.S. average rates. Although meningococcal and Tdap vaccines were not specifically emphasized in this intervention, simultaneous receipt of one or both of these vaccines with HPV may explain the increases observed. A strategy in the 4 Pillars™ Program is to offer all indicated vaccines at the time of any other vaccination. Some options for executing this strategy include offering HPV dose 1 with the same matter-of-fact tone as the two other vaccines (Tdap and meningococcal) that are simultaneously due, offering vaccination “clinics” in which only vaccinations are performed and any that are needed can be received, or pre-identifying other needed vaccines for adolescents who are scheduled to attend an influenza vaccine clinic.
Influenza vaccination among adolescents remains problematic. Pennsylvania’s reported influenza vaccination rate, which does not include Philadelphia, and the U.S. influenza vaccination rate for 2015 were each only 47%.20 However, small changes overall in influenza vaccination occurred in participating practices, up 2.3 PP overall (P<0.001). A partial explanation for smaller improvement in influenza uptake than the other vaccines may be that during the 2015–2016 influenza season, many of the practices reported late delivery of influenza vaccine – late September-early October, and then receiving only partial orders, thus narrowing the window of intervention. It is possible that many adolescents missed receiving the vaccine simply because it was not available at the time of their visits.
Size of the practice may be related to adolescent vaccination uptake because size may affect the practice’s hierarchy, responsiveness to change, and staffing structure, among other factors that have been shown to affect readiness to initiate practice change.5 In this study, size of practice was related to likelihood of Tdap and influenza vaccination. Adolescents in smaller practices were less likely to receive Tdap vaccine, but more likely to receive influenza vaccine. These findings confirm an earlier study among children in which likelihood of influenza vaccination was increased with smaller office size,21 although the definition of smaller practice was considerably larger (<5000 patients) than in the current study. However, the practice sizes in this study accounted for patients ages 11–17 years only, not all children.
Strengths and Limitations
The strengths of this study lie in the use of an intervention shown to be effective in adults22–24 and adapted for use among adolescent patients, the inclusion of five vaccines, lessons learned from the parent RCCT about ways to enhance practice engagement, and the large number of adolescents included. The participating practices were all part of a single health system using a single EMR, enabling consistency in reporting vaccine administration and subsequent vaccination data collection. Limitations include the pre-post study design, although the parent study was a RCCT and being conducted in a single geographic region, which may limit generalizability. Another limitation are the facts that the cohort was 11–17 years of age at baseline and one year older post intervention and HPV vaccination increased with age. Hence some of the vaccination increases particularly for HPV could be attributable to the aging of the population.
Conclusions
A review of practice- and community-based interventions concluded that health care settings are an important venue for improving HPV vaccination rates and that even small increases in rates observed in research studies may have large effects when broadly applied.2 Clinically and statistically significant improvements in HPV series initiation, completion, meningococcal and Tdap vaccinations were observed in primary care practices implementing the 4 Pillars™ Practice Transformation Program. Increases in vaccine uptake resulted in post intervention rates that were higher than both state and national average coverage levels.
Supplementary Material
Acknowledgments
Funding: This work was supported by a research award (Grant ID 8201807) from Merck & Co., Inc. The views expressed herein are those of those authors and not those of Merck & Co, Inc. The project described was also supported by the National Institutes of Health through Grant Numbers (UL1 RR024153) and (UL1TR001857). The funders had no role in the design, execution of the study or the analysis or interpretation of data. Additionally, this work was supported in part by a research grant from Investigator-Initiated Studies Program of Merck Sharp & Dohme Corp. The opinions expressed in this paper are those of the authors and not necessarily represent those of Merck Sharp & Dohme Corp.
The authors would like to thank the participating practices from the University of Pittsburgh CTSI Pediatric Pittnet, the FM PittNet and Community Medicine, Inc.
List of Abbreviations
- HPV
Human Papillomavirus Vaccine
- Tdap
Tetanus-pertussis-diphtheria
- 4 Pillars™ Program
The 4 Pillars™ Practice Transformation Program
- RCCT
Randomized Controlled Cluster Trial
- PP
Percentage Point
Footnotes
The manuscript was drafted by Drs. Nowalk, Lin and Zimmerman.
Clinical Trial Registry Number: NCT02165722
Conflicts of Interest: Dr. Humiston is a consultant for Hager Sharp Inc. and Pfizer, Inc. Dr. Reis has research funding from Merck & Co., Inc. and Pfizer, Inc. for this project. Drs. Zimmerman and Lin and Ms. Moehling have research funding from Sanofi Pasteur, Inc. Drs. Zimmerman, Nowalk, Lin, Mr. Raviotta and Ms. Moehling have research funding from Pfizer, Inc. and Merck & Co., Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.US Department of Health and Human Services. [Accessed May 30, 2013];Healthy People 2020. 2012 http://www.healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicid=23.
- 2.Niccolai LM, Hansen CE. Practice- and Community-Based Interventions to Increase Human Papillomavirus Vaccine Coverage: A Systematic Review. Jama Pediatr. 2015;169(7):686–692. doi: 10.1001/jamapediatrics.2015.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perkins RB, Lin M, Silliman RA, Clark JA, Hanchate A. Why Are U.S. Girls Getting Meningococcal But Not Human Papilloma Virus Vaccines? Comparison of Factors Associated with Human Papilloma Virus and Meningococcal Vaccination Among Adolescent Girls 2008 to 2012. Women's Health Issues. 2015;25(2):97–104. doi: 10.1016/j.whi.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Jeudin P, Liveright E, Del Carmen MG, Perkins RB. Race, ethnicity, and income factors impacting human papillomavirus vaccination rates. Clinical therapeutics. 2014;36(1):24–37. doi: 10.1016/j.clinthera.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Hawk M, Nowalk MP, Moehling KK, et al. Using a mixed methods approach to examine practice characteristics associated with implementation of an adult immunization intervention using the 4 Pillars™ Practice Transformation Program. Journal for Healthcare Quality. 2016 doi: 10.1097/JHQ.0000000000000071. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zimmerman RK, Moehling KK, Lin CJ, et al. Improving Adolescent HPV Vaccination in a Randomized Controlled Cluster Trial Using the 4 Pillars™ Practice Transformation Program. Vaccine. 2017;35:109–117. doi: 10.1016/j.vaccine.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meites EKA, Markowitz LE. [Accessed 12/16/16, 2016];Use of a 2-Dose Schedule for Human Papillomavirus Vaccination — Updated Recommendations of the Advisory Committee on Immunization Practices. 2016 doi: 10.15585/mmwr.mm6549a5. https://www.cdc.gov/mmwr/volumes/65/wr/mm6549a5.htm. [DOI] [PubMed]
- 8.Oldenburg B, Parcel SG. Diffusion of Innovations. In: Karen Glanz, Rimer Barbara K, Lewis Frances M., editors. Health Behavior and Health Education. 3. San Francisco: John Wiley and Sons, Inc; 2002. pp. 312–334. [Google Scholar]
- 9.Brook RH, Chassin MR, Fink A, Solomon DH, Kosecoff J, Park RE. A method for the detailed assessment of the appropriateness of medical technologies. Int J Technol Assess Health Care. 1986;2(1):53–63. doi: 10.1017/s0266462300002774. [DOI] [PubMed] [Google Scholar]
- 10.Melinkovich P, Hammer A, Staudenmaier A, Berg M. Improving pediatric immunization rates in a safety-net delivery system. Joint Commission journal on quality and patient safety / Joint Commission Resources. 2007;33(4):205–210. doi: 10.1016/s1553-7250(07)33024-9. [DOI] [PubMed] [Google Scholar]
- 11.Zimmerman RK, Moehling KK, Lin CJ, et al. Improving adolescent HPV vaccination in a randomized controlled cluster trial using the 4 Pillars™ practice Transformation Program. Vaccine. 2017;35(1):109–117. doi: 10.1016/j.vaccine.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. [Accessed 12-5-2016, 2016];Teen Vax View Interactive. 2015 https://www.cdc.gov/vaccines/imz-managers/coverage/teenvaxview/data-reports/index.html.
- 13.Widdice LE, Bernstein DI, Leonard AC, Marsolo KA, Kahn JA. Adherence to the HPV vaccine dosing intervals and factors associated with completion of 3 doses. Pediatrics. 2011;127(1):77–84. doi: 10.1542/peds.2010-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McRee AL, Brewer NT, Reiter PL, Gottlieb SL, Smith JS. The Carolina HPV immunization attitudes and beliefs scale (CHIAS): scale development and associations with intentions to vaccinate. Sexually transmitted diseases. 2010;37(4):234–239. doi: 10.1097/OLQ.0b013e3181c37e15. [DOI] [PubMed] [Google Scholar]
- 15.Marlow LA, Waller J, Wardle J. Parental attitudes to pre-pubertal HPV vaccination. Vaccine. 2007;25(11):1945–1952. doi: 10.1016/j.vaccine.2007.01.059. [DOI] [PubMed] [Google Scholar]
- 16.Brewer NT, Fazekas KI. Predictors of HPV vaccine acceptability: a theory-informed, systematic review. Prev Med. 2007;45(2–3):107–114. doi: 10.1016/j.ypmed.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Kester LM, Zimet GD, Fortenberry JD, Kahn JA, Shew ML. A national study of HPV vaccination of adolescent girls: rates, predictors, and reasons for non-vaccination. Maternal and child health journal. 2013;17(5):879–885. doi: 10.1007/s10995-012-1066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mays RM, Sturm LA, Zimet GD. Parental perspectives on vaccinating children against sexually transmitted infections. Soc Sci Med. 2004;58(7):1405–1413. doi: 10.1016/S0277-9536(03)00335-6. [DOI] [PubMed] [Google Scholar]
- 19.Pennsylvania Department of Health. [Accessed March 2, 2016];School Children Immunizations. http://www.health.pa.gov/My%20Health/Immunizations/School/Pages/default.aspx#.VtdCi_krKUk.
- 20.Centers for Disease Control and Prevention. [Accessed 12/5/2016, 2016];2015–16 Influenza season vaccination coverage estimates for local areas and territories. 2016 https://www.cdc.gov/flu/fluvaxview/local-areas-estimates-2015-16.htm.
- 21.Lin CJ, Nowalk MP, Toback SL, Ambrose CS. Factors associated with in-office influenza vaccination by US pediatric providers. BMC pediatrics. 2013;13(1):1. doi: 10.1186/1471-2431-13-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin CJ, Nowalk MP, Pavlik VN, et al. Using the 4 pillars™ practice transformation program to increase adult influenza vaccination and reduce missed opportunities in a randomized cluster trial. BMC infectious diseases. 2016;16(1):623. doi: 10.1186/s12879-016-1940-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nowalk MP, Lin CJ, Pavlik VN, et al. Using the 4 Pillars™ Practice Transformation Program to increase adult Tdap immunization in a randomized controlled cluster trial. Vaccine. 2016;34(41):5026–5033. doi: 10.1016/j.vaccine.2016.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zimmerman RK, Brown AE, Pavlik VN, et al. Using the 4 Pillars™ Practice Transformation Program to increase pneumococcal immunizations for older adults: A cluster randomized trial. J Am Geriatr Soc. 2017;65:114–122. doi: 10.1111/jgs.14451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.