Figure 1.
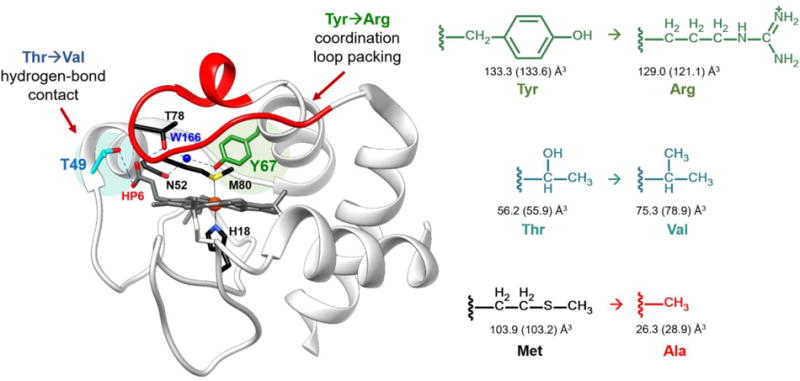
Structure of WT horse heart cyt c (PDB ID: 1HRC).6 Highlighted are the residues participating in the intraprotein hydrogen-bonding networks. A hydrogen-bond contact between the hydroxyl of Thr49 (cyan) and carboxyl group of outer-heme priopionate-6 was broken by mutating the residue to Val. Coordination loop packing was perturbed by mutating Tyr67 (green) to Arg, which also participates in the hydrogen-bonding network between Met80 and Thr78. Shown in right are the corresponding side chains of the amino acids introduced in the variants studied, with the side chain volumes (SCvol) of the amino acids shown when buried and in solution (in parentheses); SCvol = Vaa−Vgly.33
