Abstract
Strong social ties correspond with better health and well being, but the neural mechanisms linking social contact to health remain speculative. This study extends work on the social regulation of brain activity by supportive handholding in 110 participants (51 female) of diverse racial and socioeconomic origins. In addition to main effects of social regulation by handholding, we assessed the moderating effects of both perceived social support and relationship status (married, cohabiting, dating or platonic friends). Results suggest that, under threat of shock, handholding by familiar relational partners attenuates both subjective distress and activity in a network associated with salience, vigilance and regulatory self-control. Moreover, greater perceived social support corresponded with less brain activity in an extended network associated with similar processes, but only during partner handholding. In contrast, we did not observe any regulatory effects of handholding by strangers, and relationship status did not moderate the regulatory effects of partner handholding. These findings suggest that contact with a familiar relational partner is likely to attenuate subjective distress and a variety of neural responses associated with the presence of threat. This effect is likely enhanced by an individual’s expectation of the availability of support from their wider social network.
Keywords: perceived social support, attachment, handholding, threat, relationships, health
Introduction
The negative impact of poor or absent social relationships is comparable to smoking, alcohol consumption, high blood pressure and a sedentary lifestyle (Holt-Lunstad et al., 2010; House et al., 1988). Risk of all-cause death is more than twice as high for those with the fewest social ties as compared with those with the most (Berkman and Syme, 1979). There are specific risks associated with social isolation, too. For example, poor social relationships are associated with higher rates of cardiovascular disease, high blood pressure, diabetes and cancer (Ertel et al., 2009; Robles and Kiecolt-Glaser, 2003; Roper and Yorgason, 2009; Uchino, 2006). For those with strong social ties, wounds even heal more quickly (Detillion et al., 2004; Kiecolt-Glaser et al., 2005). But despite the unequivocal knowledge that strong social ties correspond with better health and well being, we still do not know how.
To be sure, several mechanisms have been proposed, few or none of which are mutually exclusive. At the most basic level, social relationships provide instrumental support—tangible assistance and resources such as help acquiring food and shelter (House, 1981). Relational partners also encourage healthy diets, exercise, and adherence to medical advice (Musick et al., 2004; Umberson et al., 2010; Waite, 1995). In contrast, the buffering hypothesis (Cohen and Wills, 1985) suggests the association between relationships and health emerges primarily from the emotional support that others provide—support that reduces, for example, the level of threat one perceives during stressful situations (Cohen, 2004). In truth, both instrumental support and stress buffering are likely to play key roles, and they may interact. For example, instrumental support may be more likely and even more efficacious when provided by individuals with whom one feels a close bond (cf. Thoits, 2011).
Several studies suggest that supportive social contact attenuates threat responding—dampening the impact of both physical and social threats on brain activity (Coan and Sbarra, 2015). For example, we have previously reported that simply holding a person’s hand while under threat may reduce activity in areas such as the prefrontal cortex, anterior cingulate cortex (ACC), caudate, and related regions putatively associated with emotional responding, top-down self control and emotion regulation, among other processes. Moreover, these reductions are likely to be moderated by things like familiarity, relationship quality, perceived mutuality, early maternal support, early neighborhood quality and trait anxiety (Coan et al., 2013a,b; Coan et al., 2006; Johnson et al., 2013; Maresh et al., 2013). Anxious children show less ventromedial prefrontal and hypothalamic activation in response to mildly threatening words when in the presence of a parent, even in the absence of supportive touch (Conner et al., 2012). Similar effects obtain when showing pictures of loved ones (Master et al., 2009; Younger et al., 2010), or even reminders of an attachment figure (Karremans et al., 2011).
Perceived social support
These effects likely reflect or indeed depend to various degrees upon the perceived availability of social resources. Interestingly, many have argued that perceived support, that is, one’s estimation of how available social resources are or will be, is not strongly associated with more objective measures of how much support a person actually receives (Reis et al., 2004). Moreover, perceived support may be more related to positive health outcomes than more objectively measured support, which suggests social support is substantially manifest at the psychological level (Reis et al., 2004; Sandler and Barrera, 1984). Others have challenged the notion that the primary mechanism linking social support to health is perceived support, presenting evidence, for example, that among older men and women social isolation was more consequential for longevity than subjective feelings of loneliness (Steptoe et al., 2013). And some have argued that the association between perceived and received support is strongest when people are under stress, or at least when the type of support needed is matched effectively to the type of support provided (Cutrona, 1990).
Relationship status
Historically, relationship status (e.g. parent, platonic friend, romantic interest, cohabiting partner, spouse) has also been emphasized as a powerful moderator of social affect regulation. The most obvious example concerns maternal/offspring attachments, which are qualitatively different than those between other conspecific pairs (Hofer, 2006). It has long been acknowledged that putative attachment bonds between caregivers (overwhelmingly mothers) and their infants are characteristic of both human and nonhuman primates (Bowlby, 1969; Harlow, 1958). As an organizational construct, attachments are identified by stereotyped behaviors including (but probably not limited to) discrimination of attachment figures from others, a demonstrable preference for attachment figures, and separation and reunion behaviors specific to attachment figures—often distress and relief, respectively (Beckes and Coan, 2015). Thus, whether distinctions are made between caregivers and strangers, mates and friends or potential mates and family members, relationship status may reflect the ordinally scaled degree of attachment between conspecifics (Mikulincer and Shaver, 2007). If true, it is important to monitor distinctions between different relationship types (e.g. romantic, platonic, etc.).
Ultimately, the current study was designed to address three issues related to our earlier work on the social regulation of neural threat responding by supportive touch. First, we report here the first large-scale attempt to broadly replicate the findings of Coan et al. (2006). The original study reported the impact of simple spousal handholding on the neural response to threat among 16 women in highly satisfactory marriages. Here, we describe a replication attempt among 110 dyads including both men and women and roughly equal amounts of individuals who are married, cohabiting, dating, or platonic friends. Moreover, we sought here to assess the moderating impact of both perceived social support and relationship status on the regulatory impact of handholding. We first hypothesized a general replication of the basic findings of Coan et al. (2006)—that simple handholding would attenuate much of the brain’s response to threat of shock, and that this attenuation would be strongest while holding hands with a familiar relational partner. Next, because perceived social support reflects the degree to which an individual believes that social support is likely, we hypothesized that higher levels of perceived social support would correspond with greater regulatory impact of handholding by anyone, but particularly by a familiar relational partner. Finally, we hypothesized that the regulatory impact of handholding among familiar relational partners would be roughly ordinally scaled, descending in magnitude from marital partners, to cohabiting partners, platonic friends and dating partners.
Materials and methods
Participants
Participants (n = 110) brought an opposite-gender partner to the scanner (14 other participants were removed from the final dataset due to equipment malfunction, problems during data collection or failure to comply with directions). Within these dyads, 27 identified as friends, 29 were dating, 27 were cohabitating and 27 were married. Of scanned participants, 54% (n = 59) were male and 46% (n = 51) were female; ages ranged from 23 to 26 years. Approximately 69% of scanned participants identified as White, 26% identified as African-American, 4% identified as Hispanic and 2% identified as Asian. Eighty-six of these participants were recruited from the Virginia Institute of Development in Adulthood (VIDA) longitudinal sample, which the Allen laboratory had been assessing for over a decade (Allen et al., 2007; McElhaney et al., 2008; Coan et al., 2013a). Because the VIDA sample did not have sufficient numbers of married participants, 24 of the 27 total married dyads (matched for demographics of the VIDA sample) were additionally drawn from the community. Participants were excluded if they were pregnant or exhibited any risk of danger in the magnetic environment of the scanner. Informed consent was obtained from both members of each dyad, and all participants were paid $160 for participation.
Measures
Multidimensional Scale of Perceived Social Support (MSPSS)
The Multidimensional Scale of Perceived Social Support (MSPSS; Zimet et al., 1988) is a 12-item questionnaire which measures three sources of perceived support: family, friends and significant others. Items are assessed using a seven-point Likert scale (1 = very strongly disagree; 7 = very strongly agree), with higher scores suggesting a higher level of perceived support. Excellent psychometric properties have been reported (Zimet et al., 1988), with internal consistency estimated to be .88 (Cronbach’s α) for the total scale and test–retest reliability estimated at ∼.85 (also α). For this study, we used total MSPSS scores. The current sample had a mean MSPSS score of 72.7 and s.d. of 13.2.
Self-Assessment Manikin (SAM) Scales (Bradley and Lang, 1994)
The SAM Scales are non-verbal measures used by participants to rate their current subjective feelings of valence and arousal. The valence scale shows pictures ranging from a smiling, happy figure to a frowning, unhappy figure, and the arousal scale shows pictures ranging from an excited, wide-eyed figure to a relaxed, sleepy figure. These scales involve choosing on a nine-point pictorial scale one’s subjective emotional valence and arousal in the moment. The valence scale is anchored by very positive (1) on one end and very negative (9) on the other. The arousal scale is anchored by not at all aroused (1) on the one end and very aroused on the other (9). Because of recording errors in this study, data are missing for seven valence ratings and three arousal ratings.
Procedure
After being screened via telephone for exclusion criteria, eligible participants visited the research MRI facility at the University of Virginia where they completed a battery of questionnaires, followed by the functional magnetic resonance imaging (fMRI) procedure. Before entering the MRI device, two Ag-AgCl shock electrodes were applied to the participant’s ankle (left or right, counterbalanced across participants). Before functional scans were obtained, high resolution anatomical scans were collected.
During functional imaging, participants viewed stimuli projected onto a screen situated behind the magnet’s bore using a mirror placed on the head coil. Participants were able to respond to stimuli through the use of a button box placed in their non-dominant hand. For this within-subjects design, all participants underwent three blocks of our threat-of-shock paradigm, in counterbalanced order. During one block, the participant held the hand of their partner; during another they held the hand of an unseen confederate of the opposite sex, and in another the participant was alone in the scanner. Each block was composed of 24 trials, 12 of which were ‘threat’ trials and 12 of which were ‘safety’ trials, presented in a variable order. Each trial was composed of a 1-s safety or threat cue, 4–10 s of an anticipation period indicated by a fixation cross, and then a small dot indicating the end of the trial. For shock trials, shocks were delivered immediately prior to the appearance of the dot indicating the trial’s end. The inter-trial interval varied from 4 to 10 s. Threat cues consisted of a red ‘X’ on a black background and indicated a 17% chance of electric shock, while safety cues consisted of a blue ‘O’ on a black background, indicating no chance of shock. Shocks were generated by an isolated physiological stimulator (Coulbourn Instruments, Allentown, PA, USA) and lasted for 20 ms at 4 mA. After each of the three blocks, participants rated their subjective assessment of their current arousal and valence using the SAM scales.
Image acquisition and analysis
Functional images were acquired using a Siemens 3.0 Tesla MAGNETOM Trio high-speed magnetic imaging device with a circularly polarized transmit/receive head coil with integrated mirror. A total of 216 functional T2*-weighted echo planar images (EPIs) sensitive to blood-oxygen-level-dependent contrasts were collected per block in volumes of 28 3.5-mm transversal echo-planar slices (1-mm slice gap) covering the whole brain (1-mm slice gap, repetition time (TR) = 2000 ms, echo time (TE) = 40 ms, flip angle = 90°, field of view (FOV) = 192, matrix = 64 × 64, voxel size = 3 × 3 × 3.5 mm). Before collection of functional images, 176 high-resolution T1-magnetization-prepared rapid-acquisition gradient echo images were acquired to determine the localization of function (1-mm slices, TR = 1900 ms, TE = 2.53 ms, flip angle = 9°, FOV = 250 mm, voxel size = 1 × 1 × 1 mm).
Data were preprocessed and analyzed using FMRIB’s Software Library (FSL) software (Version 5.0; www.fmrib.ox.ac.uk/fsl; Worsley, 1994). Motion correction involved FMRIB’s Linear Image Registration Tool and intra-modal correction algorithm tool (MCFLIRT; Jenkinson et al., 2002), with slice scan time correction and a high-pass filtering cutoff point of 100 s, removing irrelevant signals. We used BET (Smith, 2002) brain extraction, eliminating non-brain material voxels in the fMRI data, and a 5-mm full width at half minimum Gaussian kernel for smoothing. Images were registered to the Montreal Neurological Institute (MNI) space by FLIRT (Jenkinson et al., 2002). Trials in which participants received shocks (two per condition) were excluded to protect against movement artifacts, resulting in 10 total shock-threat trials per condition.
Using FEAT (FMRI Expert Analysis Tool) Version 6.00, part of FSL (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl (accessed 8 March 2017)) and time-series analysis by FILM (Worsley, 2001), our individual-level analysis of the functional data began with a threat minus safe contrast, applied separately to each handholding condition, for each subject. Data were then collapsed across the three handholding conditions using a higher level FEAT analysis employing a fixed effects model. Here, additional contrasts comparing each handholding condition in all possible combinations were employed (i.e. alone minus stranger; alone minus partner; stranger minus alone; stranger minus partner; partner minus alone; partner minus stranger).
Finally, four group-level analyses were completed. To localize the main effect of threat, the alone condition threat minus safe contrast was brought to the group level with FEAT FLAME (FMRIB's Local Analysis of Mixed Effects) 1 using a whole brain clusterwise threshold of z = 2.3 and P < .05. The threat minus safe contrast from the alone condition was used as a pre-threshold mask for all subsequent group-level analyses. Next, individual level contrasts of handholding condition were brought to the group level using Randomise (Winkler et al., 2014) and identified via threshold free cluster enhancement (TFCE; Smith and Nichols, 2009), P < .05. Additional analyses in which we used the threat minus safe contrast from the stranger and partner conditions as pre-threshold masks are included in the Supplementary materials. Randomise is a nonparametric permutation based analytical method developed to improve type I error control (see Eklund et al., 2016). TFCE detects significant clusters of voxels, controlling for the family-wise error rate, without the need to create cluster size or voxel intensity thresholds. This method of analysis is generally more conservative than analyses employing traditional parametric cluster-thresholding procedures (see also Zhang et al., 2012). Descriptive statistics were extracted as the mean percent signal change from all functionally derived regions of interest (ROIs).
To assess the impact of perceived social support, MSPSS scores were centered and included as a covariate in additional random permutation tests. To assess the impact of relationship type, relationship type was included as a group level independent variable. Specifically, we contrasted each relationship type (married, cohabiting, dating, friend) from the average of the other three. For example, when testing for a marriage effect, we subtracted the average of the cohabiting, dating and friend groups from the married group. These contrasts were used to detect whether relationship type moderated the effect of handholding on threat activity.
Results
Subjective measures
To investigate the subjective effects of handholding, we conducted within-subjects ANOVAs on SAM ratings of arousal and valence. Self-reported levels of arousal did not differ across the handholding conditions, F(2, 104) = .55, P = .58 (Malone = 3.1, SEalone = .19; Mstranger = 3.2, SEstranger = .19; Mpartner = 3.2, SEpartner = .18. However, levels of valence did differ across handholding conditions, F(2, 99) = 8.5, P < .001, with participants reporting more negativity in the Stranger (M = 5.9, SE = .19) condition than in both the Partner (M = 5.0, SE = .20), t(101) = 4.2, P < .001, and Alone (M = 5.3, SE = .20) conditions, t(103) = 2.5, P = .01. Participants showed a non-significant trend toward reporting more negativity in the Alone condition than in the Partner condition, t(101) = 1.8, P = .08. When predicting arousal, no significant interactions emerged between handholding and MSPSS (F(2, 100) = .94, P = .39) or handholding and relationship status (F(6, 202) = .55, P = .58). Similarly, when predicting valence, no significant interactions emerged between handholding and MSPSS (F(2, 95) = .33, P = .72) or handholding and relationship status (F(6, 202) = .77, P = .59).
Main effects of threat
Group level analyses of the threat minus safe contrast indicated threat responses across putative salience and executive networks (cf. Bressler and Menon, 2010) in all three handholding conditions (see Table 1, Supplementary Figure S1, and Supplementary Table S1). Activations peaked in the junction between the anterior insula, orbitofrontal cortex and operculum, extending broadly into ventral and dorsal lateral frontal cortices, cingulate cortex, dorsal and ventral striatum, thalamus, hypothalamus and brainstem, posterior parietal cortex, somatosensory and motor cortices and precuneus. Per Coan et al. (2006), a mask derived from threat minus safe contrasts during the alone condition was used to compare these activations across handholding conditions (alone, stranger, partner). Supplementary materials contain similar analyses drawing on partner and stranger threat minus safe masks.
Table 1.
Significant clusters of activity for the threat minus safe contrast in the alone condition and local maxima
| MNI coordinates | ||||||
|---|---|---|---|---|---|---|
| Structural location | Cluster size in voxels | Estimated cluster P-value | Z-score | X | Y | Z |
| Alone condition | ||||||
| Threat minus safe cluster | 47 850 | 0 | ||||
| Orbitofrontal cortex | 9.43 | 36 | 28 | −6 | ||
| Supramarginal gyrus | 9.14 | 58 | −42 | 32 | ||
| Anterior insula | 9.02 | 36 | 22 | −2 | ||
| Orbitofrontal cortex | 8.9 | −32 | 26 | −6 | ||
| Anterior insula | 8.73 | −36 | 18 | −4 | ||
| Posterior cingulate cortex | 8.61 | 2 | −22 | 24 | ||
| Precuneus cortex | 511 | .02 | 6.19 | 12 | −72 | 38 |
Main effects of handholding
A series of pairwise comparisons between each handholding condition were carried out. Effects emerged in the Alone minus Partner contrast and the Stranger minus Partner contrast, each of which is decomposed below. Only contrasts with significant activation differences are reported. No effects emerged in any other contrast.
Alone minus Partner
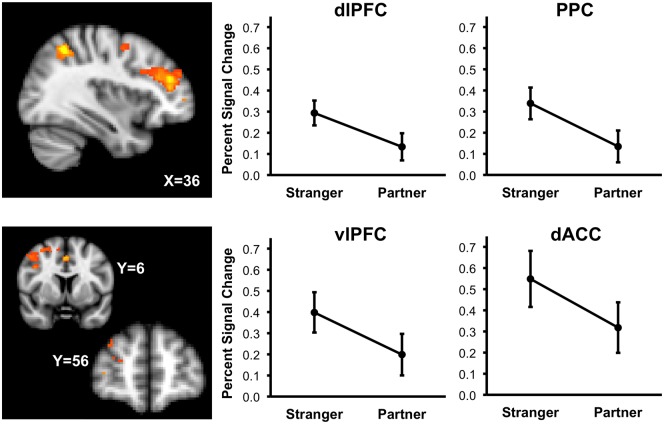
The alone minus partner contrast revealed less activity during partner handholding in the right dorsolateral prefrontal cortex (dlPFC; 32, 30, 22; alone: M = .28, SE = .03; partner: M = .13, SE = .03) and posterior cingulate cortex (PCC; -2, -22, 22; alone: M = .39, SE = .04; partner: M = .17, SE = .04). Details of these effects are provided in Table 2 and Figure 1.
Table 2.
Significant clusters of activity for the main effect of handholding on the threat minus safe contrast and interaction between handholding condition and MSPSS
| MNI coordinates | |||||
|---|---|---|---|---|---|
| Structural location | Cluster size in voxels | Z-max | X | Y | Z |
| Alone—partner main effect | |||||
| dlPFC | 116 | 4.54 | 32 | 30 | 22 |
| PCC | 13 | 4.04 | −2 | −22 | 22 |
| Stranger—partner main effects | |||||
| dlPFC | 1350 | 5.19 | 32 | 40 | 20 |
| PPC | 403 | 5.08 | 36 | −54 | 50 |
| dACC | 81 | 4.08 | 0 | 6 | 46 |
| vlPFC | 3 | 3.48 | 36 | 56 | 4 |
| Stranger—partner with MSPSS interaction effects | |||||
| Inferior frontal gyrus | 117 | 4.8 | 56 | 14 | 8 |
| Operculum | 60 | 4.57 | 52 | 0 | 12 |
| Right putamen | 27 | 3.84 | 30 | 4 | 2 |
| Right putamen | 25 | 4.18 | 28 | 0 | 20 |
| Inferior frontal gyrus | 16 | 4.5 | 52 | 26 | −4 |
| Middle frontal gyrus | 9 | 3.72 | 32 | 12 | 28 |
| Right caudate | 3 | 3.46 | 18 | 0 | 12 |
Fig. 1.
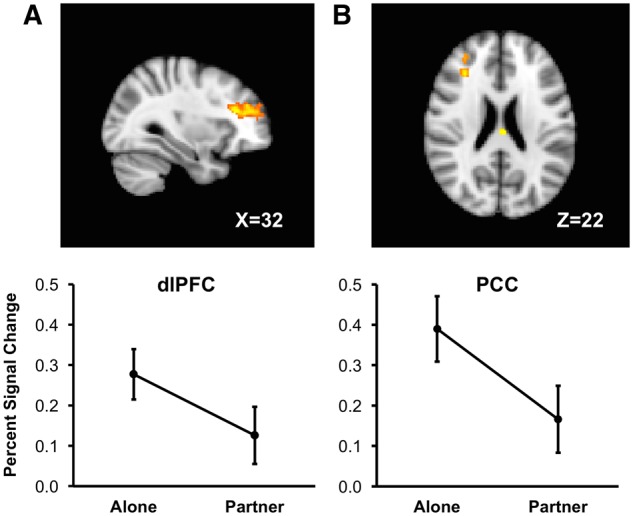
Column A: A sagittal slice (X = 32) showing a cluster in the dorsolateral prefrontal cortex (dlPFC), with graph (below) depicting average threat minus safe percent signal change in dlPFC across alone and partner handholding conditions, including 95% confidence intervals. Column B: An axial slice (Z =22) showing a cluster in the posterior cingulate cortex (PCC) and part of the dlPFC cluster, with graph (below) depicting average threat minus safe percent signal change in PCC across alone and partner handholding conditions, with 95% confidence intervals.
Stranger minus Partner
The Stranger minus Partner contrast revealed less activity during partner handholding in the right dlPFC (32, 40, 20; stranger: M = .29, SE = .03; partner: M = .13, SE = .02), posterior parietal cortex (PPC; 36, -54, 50; stranger: M = .34, SE = .04; partner: M = .13, SE = .03), dorsal anterior cingulate cortex (dACC; 0, 6, 46; stranger: M = .55, SE = .06; partner: M = .32, SE = .05) and right ventrolateral prefrontal cortex (vlPFC; 36, 56, 4; stranger: M = .40, SE = .05; partner: M = .20, SE = .05). For details see Table 2 and Figure 2.
Fig. 2.
Probability maps depicting areas where percent signal change in the threat minus safe contrast is greater during Stranger handholding than Partner handholding. Sagittal slice (X = 36) depicts areas of dorsal lateral prefrontal cortex (dlPFC) and posterior parietal cortex (PPC). Coronal slices (Y = 6) depict areas of the anterior cingulate cortex (ACC) and the middle frontal gyrus. Graphs depict average threat minus safe percent signal change across stranger and partner handholding conditions, with 95% confidence intervals, in the dlPFC, PPC, vlPFC and dACC.
Handholding by perceived social support
In order to test whether perception of social support interacted with handholding conditions to determine the magnitude of threat responding, all combinations of handholding condition contrasts were calculated with MSPSS entered as a covariate. MSPSS scores interacted with the Stranger minus Partner contrast, but no other significant effects were detected.
Stranger minus Partner with MSPSS
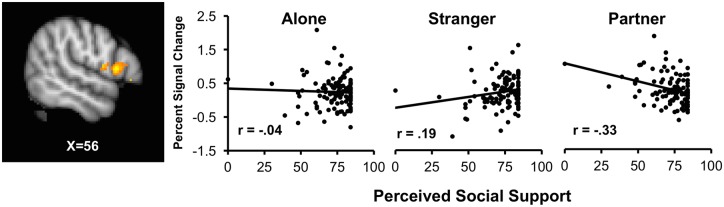
MSPSS scores significantly interacted with the Stranger minus Partner contrast in two right inferior frontal gyrus (IFG) clusters (56, 14, 8; 62, 26, -4), two right putamen clusters (30, 4, 2; 28, 0, 20), right frontal operculum (52, 0, 12), right middle frontal gyrus (32, 12, 28) and right caudate (18, 0, 12). Decomposition of these interactions revealed that higher perceived social support corresponded with greater activity during Stranger handholding and decreased threat responding during Partner handholding. Figure 3 depicts correlations between MSPSS and threat-related activity in the IFG while alone (r = -.04, P = .68), stranger handholding (r = .19, P = .05) and partner handholding (r = -.33, P < .001).
Fig. 3.
Sagittal slice (X = 56) depicting a probability map in the inferior frontal gyrus (IFG), where the percent signal change in the threat minus safe contrast is greater during stranger handholding than partner handholding as a function of perceived social support (MSPSS, centered on the mean). Scatterplots show the association between percent signal change in the threat-safe contrast and MSPSS for each handholding condition. As perceived social support increases, threat minus related activation in IFG increases during the stranger handholding (r = .19, P = .05), but decreases during partner handholding (r = -.33, P < .001). Notably one participant was an outlier on MSPSS, but results did not change when this participant removed.
Handholding by relationship status
In order to test whether relationship status interacted with handholding conditions to determine the magnitude of threat responding, all combinations of handholding condition contrasts were calculated with relationship status entered as a between-subjects factor. Relationship status did not reach threshold as a predictor of brain activity during threat of shock as a function of handholding.
Discussion
Social regulation of the brain’s multifaceted response to threat of shock—even via simple handholding—is robust. Even under threat, several regions, notably the dlPFC and posterior cingulate, that were less active during spousal handholding in Coan et al. (2006) were also less active during handholding by relational partners in the current study. As with the earlier study, subjective valence ratings were significantly improved by partner handholding, though subjective arousal ratings—a far smaller effect in the original study—were not. And echoing the relationship adjustment findings reported by Coan et al. (2006), greater perceived social support corresponded with less activity, in the context of threat, in a variety of neural regions, but only during partner handholding. No similar association obtained during stranger handholding or while alone in the scanner.
Amid these confirmations, however, were some noteworthy surprises. First, we did not observe any regulatory effects of handholding by strangers. Indeed, if anything, we observed the opposite—that stranger handholding may have potentiated many of the neural responses following exposure to threat. Second, relationship status, whether the hand holder was a spouse, a cohabiting partner, a dating partner or even a friend, did not appear to matter much for the regulatory effects of partner hand holding. These findings and their implications are discussed in greater detail below.
Regulation of the threat response by handholding
Regulation by relational partners
We found that holding hands with a familiar partner attenuated the brain’s response to shock threat when compared with holding hands with a stranger or anticipating the shock while alone. Specifically, relative to either being alone in the scanner or while holding hands with a stranger, we observed diminished activations in the dACC, the PCC, the posterior parietal cortex (PPC), the right vlPFC and the right dlPFC, while holding hands with a relational partner. These regions are notable for at least two reasons: First, they are similar to those attenuated by spouse handholding in Coan et al. (2006), a study of only 16 happily married women. Second, the consistency of these effects hints that social affect regulation may target a relatively specific network of regions implicated in the brain’s overall response to threat, including regions related to the mobilization of attentional and physiological resources and—we think critically—those linked to inhibitory control and the effortful regulation of emotion (cf. Reeck et al., 2016). Social baseline theory (see Beckes and Coan, 2011; Coan and Maresh, 2014; Coan and Sbarra, 2015) suggests that social resources signal less of a need for attention to potential threats, which in turn likely precludes the need for subsequent or concomitant top-down self-regulatory activity. The findings reported here are consistent with this perspective.
Regulation by strangers
In Coan et al. (2006), handholding by strangers resulted in a more limited level of regulation of the brain’s response to the shock threat, compared with handholding by partner. In another test of this handholding paradigm, stranger handholding caused limited attenuation of the brain’s response to shock threat after, but not before, marital therapy (Johnson et al., 2013). Here, handholding by strangers was unexpectedly ineffective. Indeed, the alone and stranger handholding conditions were not generally different. Differences between the current and original studies in terms of stranger handholding effects may reflect a number of things. To start, the current sample is substantially more representative of the general population, consisting of a much more racially and socioeconomically diverse group of friends, spouses, cohabiting partners and dating partners, all from the Charlottesville, VA area. In contrast, the original sample consisted of almost exclusively white, highly satisfied married couples from Madison, WI. Within the sample discussed here, we have already observed that lower adolescent neighborhood quality corresponded with increased prefrontal and dACC activation to a social exclusion task (Gonzalez et al., 2015). Others have reported that lower social status is linked to threat-related appraisals of ambiguous social stimuli (e.g. Chen and Paterson, 2006), and indeed even fear of strangers (Dallago et al., 2009). And whatever else is true, strangers are ‘people who have never met before, will never meet again, and … have no acquaintances in common’ (Clark-Polner and Clark, 2014, p. 2), which may limit their effectiveness as a source of emotional support even under the best circumstances.
We have described elsewhere how a putative neural measure of self/other overlap suggests that although the brain encodes ‘friend’ in ways that are similar to ‘self,’ no such (or substantially less) similarity exists between ‘stranger’ and ‘self’ (Beckes et al., 2013). Social baseline theory suggests this self/other overlap may in fact mediate expectations of support (Coan and Sbarra, 2015). It is perhaps more surprising, then, that strangers have resulted in attenuation of the brain’s response to shock threat in other samples. In the aggregate, we feel the evidence does suggest that although the supportive effect of stranger handholding is clearly limited, stranger handholding is nevertheless likely to regulate aspects of the brain’s response to shock threat in some people, some of the time. Given the potential utility of strangers in regulating negative affect, more work on the moderators of stranger handholding is warranted.
Moderators and mechanisms
Perceived social support
Self-reported levels of perceived social support moderated the effect of handholding condition. Specifically, greater perceived social support corresponded with diminished activations in the portions of the IFG, right putamen, right operculum, right middle frontal gyrus and right caudate, but only during partner handholding, suggesting that individuals who perceive a supportive social network may be more receptive to support from someone familiar to them. Moreover, these interactions suggest perceived social support may target—and attenuate—an extended socially regulated network associated with the cognitive control and associative learning that threat responding often entails.
These effects are in line with decades of work on how perceived social support may buffer the impact of negative life events (e.g. Cohen and Wills, 1985). Throughout this work, the question of whether received or perceived support is more consequential has occasionally arisen. At least one recent answer to this question is that the two forms of support interact in much the same way we have observed in the present study: that perceived support works in part by potentiating the impact of received support (Melrose et al., 2015). Less clear is why greater perceived social support also corresponded with more, not less, threat responding during stranger handholding. This may suggest that individuals in the current study who perceived greater levels of support by family, friends and significant others (which is what the MSPSS targets) may also have a greater preference for support from individuals who are familiar. Alternatively, these individuals simply may not expect strangers to be of much help.
Relationship status
As reviewed above, the impact of familiarity on the regulatory effect of handholding was robust. Holding hands with a relational partner resulted in substantially attenuated threat-related activations, while holding hands with a stranger did not. But this handholding effect was not moderated by the type of relational partner with whom our participants visited the laboratory. Whether one was holding hands with a spouse, a cohabiting partner, a dating partner or a friend made no detectable difference.
This outcome rests uneasily next to perspectives emphasizing the unique regulatory contribution of ‘attachment figures,’ or research suggesting a specific ‘marriage effect’ on everything from general mortality (Frisch and Simonson, 2013) to overall life satisfaction (Stack and Eshleman, 1998). That said, more recent evidence suggests that the effects of marriage on life satisfaction (at least) may depend non-trivially on the degree to which individuals in a romantic union regard each other as friends, whether married or cohabiting. That is, after adjusting for pre-relationship levels of happiness, differences between marriage and cohabitation largely disappear, while increases in happiness attributable to increases in perceived degree of friendship, regardless of relationship type, remain (Grover and Helliwell, 2014). Moreover, although secure attachment is positively associated with the likelihood of being married (Mickelson et al., 1997), marriage is far from a necessary ingredient in the formation and maintenance of an adult attachment bond, either in principle or empirical fact (Coan, 2016; Doherty and Feeney, 2004).
Social baseline theory holds that individuals in effect ‘outsource’ various neural activities to available support providers when and where it is possible to do so (Beckes and Coan, 2011; Coan and Maresh, 2014). Indeed, social baseline theory predicts processing devoted to threat vigilance and self-regulation should be particularly important targets of regulation by support providers, in part because of the manifold opportunity costs threat vigilance and self-regulation efforts entail. In many species, a second pair of eyes can reduce the need for vigilance (Bertram, 1980), and presumably its concomitant anxiety, mobilization of resources and self-regulatory effort.
Of course, whether one outsources vigilance to a relational partner may depend on the degree to which that relational partner can be trusted to devote vigilance processing on one’s behalf, raising again the distinction between familiar others and strangers, but also the question of how such trust is formed and maintained. Past support from a given individual is probably a good indication that support will be provided in the future (Beckes et al., 2016), which may account in part for a reliable difference in the handholding effect between many different types of familiar relational partners and strangers (e.g. Beckes and Coan, 2015; Maisel and Gable, 2009; Reis, 2007; Thoits, 2011). Of course, a remaining and non-trivial caveat on the question of moderation by relationship status concerns statistical power. With only 110 participants spread across four roughly equal groups, we run a real risk here of a false negative (Vadillo et al., 2016). Thus, our confidence in the absence of a relationship status effect is not high and, as ever, awaits additional research.
Familiarity and the self
According to social baseline theory, individuals in a wide variety of relational partnerships share goals and the efforts—both physiological and cognitive—required to meet them (Coan and Sbarra, 2015). This sharing of goals in turn influences the way each individual budgets his or her personal resources in meeting those demands. Thus, and as mentioned above, individuals can ‘outsource’ part of the labor associated with meeting a given situational demand to their social networks, or pool their resources with others in such a way that effectively achieves the same outcome: reducing the cost (in personal resources) of achieving the goal or meeting the demand. Social baseline theory suggests this is achieved in part by altering what the brain construes as the ‘self,’ expanding the self to include others in the social network or relationship. This theoretical formulation is consistent with observations (mentioned above) we have made suggesting that the brain responds to threat stimuli directed at a friend very similarly to how it responds to threats directed at the self, while no such similarity obtains when the threat is directed at a stranger (Beckes et al., 2013).
Just this sort of ‘involuntary breach of individual separateness’ (Langer, 1974, p. 129) has been observed by many others as well, both behaviorally (Andersen and Chen, 2002; Aron and Aron, 1996; Aron and Fraley, 1999; Mashek et al., 2003), and at the neural level (Decety and Sommerville, 2003; de Vignemont and Singer, 2006; Hein and Singer, 2008). If individuals construe relational partners as extensions of the self—as extensions of what the self is capable of—then part of the effect of proximity could be to cause the brain to attenuate its efforts toward, in this case, the vigilance and top-down regulatory control that follows the threat of shock, all on the assumption that the relational partner can be counted on to fill in any attentional or regulatory gaps.
Conclusion
Physical contact with a familiar other attenuated activity in brain regions associated with vigilance, salience detection, inhibitory control and emotion regulation, all in the context of shock threat, and these effects were enhanced by an individual’s expectation of support from their wider social network. Physical contact with a stranger, however, was not similarly effective, warranting further research into potential trait and situational moderators of the impact of stranger-provided support. As with our previous work, it is important to note that the findings reported here may not generalize to discordant relationships (e.g. Johnson et al., 2013) or to individuals suffering from low levels of perceived social support. Moreover, the social regulation of threat responses is likely to also vary as a partial function of other trait-like characteristics and situations. For example, developmental levels of neighborhood quality and maternal support may modulate the impact of supportive handholding (Coan et al., 2013a), as may interpersonal mutuality (Coan et al., 2013b), trait anxiety (Maresh et al., 2013) and attachment security (Ognibene and Collins, 1998; Anan and Barnette, 1999). In the meantime, results presented here provide extended evidence that the neural systems through which multiple behavioral and cognitive responses to threat are mediated are attenuated by the presence of familiar relational partners, and that subjective expectations that support is available when needed enhances this attenuation.
Supplementary Material
Acknowledgements
This research was supported by a National Institute of Mental Health grant, Award Number R01MH080725, to J.A.C. We thank Joseph P. Allen, Zoe Englander, Sara Medina-Devilliers, Alexander Tatum and Cat Thrasher for their assistance in preparing and conducting this study.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Allen J.P., Porter M., McFarland C., McElhaney K.B., Marsh P. (2007). The relation of attachment security to adolescents’ paternal and peer relationships, depression, and externalizing behavior. Child Development, 78(4), 1222–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen S.M., Chen S. (2002). The relational self: An interpersonal social-cognitive theory. Psychological Review, 109(4), 619–45. [DOI] [PubMed] [Google Scholar]
- Anan R.M., Barnett D. (1999). Perceived social support mediates between prior attachment and subsequent adjustment: a study of urban African American children. Developmental Psychology, 35(5), 1210.. [DOI] [PubMed] [Google Scholar]
- Aron E.N., Aron A. (1996). Love and expansion of the self: The state of the model. Personal Relationships, 3(1), 45–48. [Google Scholar]
- Aron A., Fraley B. (1999). Relationship Closeness as Including Other in the Self: Cognitive Underpinnings and Measures. Social Cognition, 17(2), 140–60. [Google Scholar]
- Beckes L., Coan J.A. (2011). Social baseline theory: the role of social proximity in emotion and economy of action. Social and Personality Psychology Compass, 5(12), 976–88. [Google Scholar]
- Beckes L., Coan J.A. (2015). The distress-relief dynamic in attachment bonding In: Zayas V., Hazan C., editors. Bases of Adult Attachment: From Brain to Mind to Behavior, New York: Springer Press, pp. 11–33. [Google Scholar]
- Beckes L., Coan J.A., Hasselmo K. (2013). Familiarity promotes the blurring of self and other in the neural representation of threat. Social Cognitive and Affective Neuroscience, 8(6), 670–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckes L., Simons K., Lewis D., Le A., Edwards W.L. (2016). Desperately seeking support: negative reinforcement schedules in the formation of adult attachment associations. Social Psychological and Personality Science, 8, 229–38. [Google Scholar]
- Berkman L.F., Syme S.L. (1979). Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. American Journal of Epidemiology, 109(2), 186–204. [DOI] [PubMed] [Google Scholar]
- Bertram B.C.R. (1980). Vigilance and group size in ostriches. Animal Behavior, 28, 278–86. [Google Scholar]
- Bowlby J. (1969). Attachment, Vol. 1 of Attachment and Loss New York: Basic Books. [Google Scholar]
- Bradley M.M., Lang P.J. (1994). Measuring emotion: the self-assessment manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry, 25(1), 49–59. [DOI] [PubMed] [Google Scholar]
- Bressler S.L., Menon V. (2010). Large-scale brain networks in cognition: emerging methods and principles. Trends in Cognitive Sciences, 14(6), 277–90. [DOI] [PubMed] [Google Scholar]
- Chen E., Paterson L.Q. (2006). Neighborhood, family, and subjective socioeconomic status: how do they relate to adolescent health? Health Psychology, 25(6), 704–14. [DOI] [PubMed] [Google Scholar]
- Clark-Polner E., Clark M.S. (2014). Understanding and accounting for relational context is critical for social neuroscience. Frontiers in Human Neuroscience, 8, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan J.A. (2016). Toward a neuroscience of attachment In: Cassidy J., Shaver P.R., editors. Handbook of Attachment: Theory, Research, and Clinical Applications, 3rd edn, New York: Guilford Press, pp. 242–69. [Google Scholar]
- Coan J.A., Beckes L., Allen J.P. (2013a). Childhood maternal support and social capital moderate the regulatory impact of social relationships in adulthood. International Journal of Psychophysiology, 88(3), 224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan J.A., Kasle S., Jackson A., Schaefer H.S., Davidson R.J. (2013b). Mutuality and the social regulation of neural threat responding. Attachment & Human Development, 15(3), 303–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan J.A., Maresh E.L. (2014). Social baseline theory and the social regulation of emotion In J.J. Gross (ed.), The Handbook of Emotion Regulation, 2nd Edition (pp. 221–36). New York, NY, USA: The Guilford Press. [Google Scholar]
- Coan J.A., Sbarra D.A. (2015). Social baseline theory: the social regulation of risk and effort. Current Opinion in Psychology, 1, 87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan J.A., Schaefer H.S., Davidson R.J. (2006). Lending a hand social regulation of the neural response to threat. Psychological Science, 17(12), 1032–9. [DOI] [PubMed] [Google Scholar]
- Cohen S. (2004). Social relationships and health. American Psychologist, 59(8), 676–84. [DOI] [PubMed] [Google Scholar]
- Cohen S., Wills T.A. (1985). Stress, social support, and the buffering hypothesis. Psychological Bulletin, 98(2), 310–57. [PubMed] [Google Scholar]
- Conner O.L., Siegle G.J., McFarland A.M., et al. (2012). Mom-it helps when you're right here! Attenuation of neural stress markers in anxious youths whose caregivers are present during fMRI. PLoS ONE, 7(12), e50680.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutrona C.E. (1990). Stress and Social Support—in Search of Optimal Matching. Journal of Social and Clinical Psychology, 9, 3–14. [Google Scholar]
- Dallago L., Perkins D.D., Santinello M., Boyce W., Molcho M., Morgan A. (2009). Adolescent place attachment, social capital, and perceived safety: a comparison of 13 countries. American Journal of Community Psychology, 44(1–2), 148–60. [DOI] [PubMed] [Google Scholar]
- Decety J., Sommerville J.A. (2003). Shared representations between self and other: a social cognitive neuroscience view. Trends in Cognitive Sciences, 7(12), 527–33. [DOI] [PubMed] [Google Scholar]
- Detillion C.E., Craft T.K., Glasper E.R., Prendergast B.J., DeVries A.C. (2004). Social facilitation of wound healing. Psychoneuroendocrinology, 29(8), 1004–11. [DOI] [PubMed] [Google Scholar]
- Doherty N.A., Feeney J.A. (2004). The composition of attachment networks throughout the adult years. Personal Relationships, 11, 469–88. [Google Scholar]
- Ertel K.A., Glymour M.M., Berkman L.F. (2009). Social networks and health: a life course perspective integrating observational and experimental evidence. Journal of Social and Personal Relationships, 26(1), 73–92. [Google Scholar]
- Frisch M., Simonsen J. (2013). Marriage, cohabitation and mortality in Denmark: national cohort study of 6.5 million persons followed for up to three decades (1982–2011). International Journal of Epidemiology, 42, 559–78. [DOI] [PubMed] [Google Scholar]
- Gonzalez M.Z., Beckes L., Chango J., Allen J.P., Coan J.A. (2015). Adolescent neighborhood quality predicts adult dACC response to social exclusion. Social Cognitive and Affective Neuroscience, 10(7), 921–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover S., Helliwell J.F. (2014). How's Life at Home? New Evidence on Marriage and the Set Point for Happiness (No. w20794). National Bureau of Economic Research.
- Harlow H.F. (1958). The nature of love. American Psychologist, 13(12), 673.. [DOI] [PubMed] [Google Scholar]
- Hein G., Singer T. (2008). I feel how you feel but not always: the empathic brain and its modulation. Current Opinion in Neurobiology, 18(2), 153–8. [DOI] [PubMed] [Google Scholar]
- Hofer M.A. (2006). Psychobiological roots of early attachment. Current Directions in Psychological Science, 15(2), 84–8. [Google Scholar]
- Holt-Lunstad J., Smith T.B., Layton J.B. (2010). Social relationships and mortality risk: a meta-analytic review. PLoS Medicine, 7(7), e1000316.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House J.S. (1981). Work Stress and Social Support. MA: Addison-Wesley Publishing Company Reading.
- House J.S., Landis K.R., Umberson D. (1988). Social relationships and health. Science, 241(4865), 540–5. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage, 17(2), 825–41. [DOI] [PubMed] [Google Scholar]
- Johnson, S.M., Burgess Moser, M., Beckes, L., et al. (2013). Soothing the threatened brain: leveraging contact comfort with emotionally focused therapy. PLoS ONE, 8(11), e79314. [DOI] [PMC free article] [PubMed]
- Karremans J.C., Heslenfeld D.J., van Dillen L.F., Van Lange P.A. (2011). Secure attachment partners attenuate neural responses to social exclusion: an fMRI investigation. International Journal of Psychophysiology, 81(1), 44–50. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser J.K., Loving T.J., Stowell J.R., et al. (2005). Hostile marital interactions, proinflammatory cytokine production, and wound healing. Archives of General Psychiatry, 62(12), 1377–84. [DOI] [PubMed] [Google Scholar]
- Maisel N.C., Gable S.L. (2009). The paradox of received social support: the importance of responsiveness. Psychological Science, 20(8), 928–32. [DOI] [PubMed] [Google Scholar]
- Mashek, D.J., Aron, A., Boncimino, M. (2003). Confusions of self with close others. Personality & Social Psychology Bulletin, 29(3), 382–92. [DOI] [PubMed]
- Master S.L., Eisenberger N.I., Taylor S.E., Naliboff B.D., Shirinyan D., Lieberman M.D. (2009). A picture's worth partner photographs reduce experimentally induced pain. Psychological Science, 20(11), 1316–8. [DOI] [PubMed] [Google Scholar]
- Maresh E.L., Beckes L., Coan J.A. (2013). The social regulation of threat-related attentional disengagement in highly anxious individuals. Frontiers in Human Neuroscience, 7, 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhaney K.B., Antonishak J., Allen J.P. (2008). “They like me, they like me not”: popularity and adolescents’ perceptions of acceptance predicting social functioning over time. Child Development, 79, 720–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melrose K.L., Brown G.D., Wood A.M. (2015). When is received social support related to perceived support and well-being? When it is needed. Personality and Individual Differences, 77, 97–105. [Google Scholar]
- Mickelson K.D., Kessler R.C., Shaver P.R. (1997). Adult attachment in a nationally representative sample. Journal of Personality and Social Psychology, 73, 1092.. [DOI] [PubMed] [Google Scholar]
- Mikulincer M., Shaver P.R. (2007). Attachment in Adulthood: Structure, Dynamics, and Change. New York: Guilford Press. [Google Scholar]
- Musick M.A., House J.S., Williams D.R. (2004). Attendance at religious services and mortality in a national sample. Journal of Health and Social Behavior, 45(2), 198–213. [DOI] [PubMed] [Google Scholar]
- Ognibene T.C., Collins N.L. (1998). Adult attachment styles, perceived social support and coping strategies. Journal of Social and Personal Relationships, 15(3), 323–45. [Google Scholar]
- Reeck C., Ames D.R., Ochsner K.N. (2016). The social regulation of emotion: an integrative, cross-disciplinary model. Trends in Cognitive Sciences, 20(1), 47–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis H.T. (2007). Steps toward the ripening of relationship science. Personal Relationships, 14(1), 1–23. [Google Scholar]
- Reis H.T., Clark M.S., Holmes J.G. (2004). Perceived partner responsiveness as an organizing construct in the study of intimacy and closeness. In D. Mashek, and A. Aron, (eds), The handbook of closeness and intimacy (pp. 201–25). Mahwah, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Robles T.F., Kiecolt-Glaser J.K. (2003). The physiology of marriage: pathways to health. Physiology & Behavior, 79(3), 409–16. [DOI] [PubMed] [Google Scholar]
- Roper S.O., Yorgason J.B. (2009). Older adults with diabetes and osteoarthritis and their spouses: effects of activity limitations, marital happiness, and social contacts on partners' daily mood. Family Relations: An Interdisciplinary Journal of Applied Family Studies, 58(4), 460–74. [Google Scholar]
- Sandler I.N., Barrera M. (1984). Toward a multimethod approach to assessing the effects of social support. American Journal of Community Psychology, 12(1), 37–52. [DOI] [PubMed] [Google Scholar]
- Smith S.M. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17(3), 143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Nichols T.E. (2009). Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage, 44(1), 83–98. [DOI] [PubMed] [Google Scholar]
- Stack S., Eshleman J.R. (1998). Marital status and happiness: a 17-nation study. Journal of Marriage and the Family, 60, 527–36. [Google Scholar]
- Steptoe A., Shankar A., Demakakos P., Wardle J. (2013). Social isolation, loneliness, and all-cause mortality in older men and women. Proceedings of the National Academy of Sciences of the United States of America, 110(15), 5797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoits P.A. (2011). Mechanisms linking social ties and support to physical and mental health. Journal of Health and Social Behavior, 52(2), 145–61. [DOI] [PubMed] [Google Scholar]
- Uchino B.N. (2006). Social support and health: a review of physiological processes potentially underlying links to disease outcomes. Journal of Behavioral Medicine, 29(4), 377–87. [DOI] [PubMed] [Google Scholar]
- Umberson D., Crosnoe R., Reczek C. (2010). Social relationships and health behavior across life course. Annual Review of Sociology, 36, 139.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadillo M.A., Konstantinidis E., Shanks D.R. (2016). Underpowered samples, false negatives, and unconscious learning. Psychonomic Bulletin & Review, 23, 87–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vignemont, F., Singer, T. (2006). The empathic brain: how, when and why? Trends in Cognitive Sciences, 10(10), 435–41. [DOI] [PubMed]
- Waite L.J. (1995). Does marriage matter? Demography, 32(4), 483–507. [PubMed] [Google Scholar]
- Worsley K.J. (1994). Local maxima and the expected Euler characteristic of excursion sets of χ2, F and t fields. Advances in Applied Probability, 26, 13–42. [Google Scholar]
- Worsley K.J. (2001). Statistical analysis of activation images. Functional MRI: An Introduction to Methods, 14, 251–70. [Google Scholar]
- Younger J., Aron A., Parke S., Chatterjee N., Mackey S. (2010). Viewing pictures of a romantic partner reduces experimental pain: involvement of neural reward systems. PLoS ONE, 5(10), e13309.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, T., Li, F., Beckes, L., Brown, C., Coan, J.A. (2012). Nonparametric inference of the hemodynamic response using multi-subject fMRI data. NeuroImage, 63(3), 1754–65. [DOI] [PubMed]
- Zimet G.D., Dahlem N.W., Zimet S.G., Farley G.K. (1988). The multidimensional scale of perceived social support. Journal of Personality Assessment, 52(1), 30–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.