Figure 4.
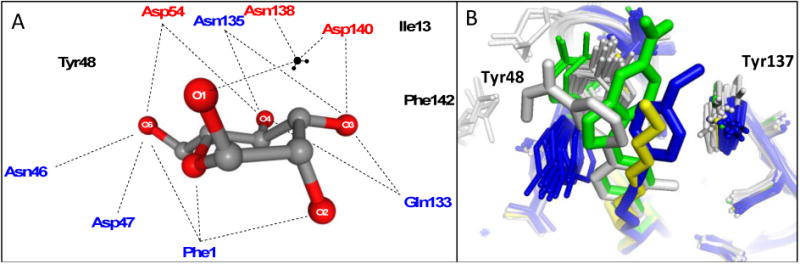
A. Schematic of residues in the polar mannose-binding pocket of the FimH lectin domain highlighting the extensive network of electrostatic and H-binding interactions of α-D-mannose with FimH. These interactions are responsible for the exquisite stereochemical specificity of FimH-containing bacteria for mannose. B. Binding pocket overlay of all reported FimH-mannoside X-ray structures showing the varied conformations of the tyrosine gate, with Tyr48 in grey (closed), blue (open) and green (open twisted), and the Tyr137 position invariant. Representative ligands shown are heptyl mannoside (yellow; 4LOV), 29 (green; 4X5Q), 18b (blue; 4AV0), and 25 (grey; 5F3F).
