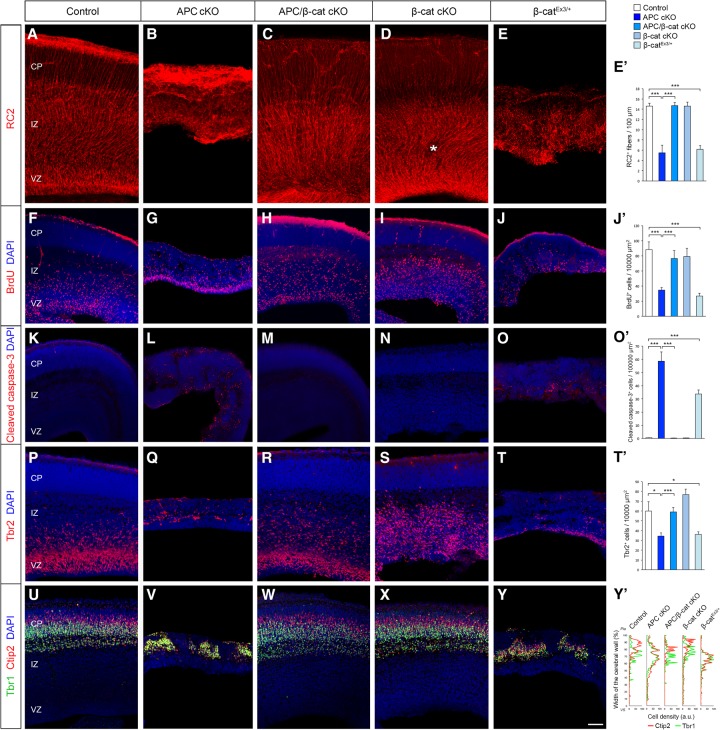
Figure 2.
APC-mediated β-catenin signaling is essential for cortical progenitor development and cerebral cortical formation. Loss of APC in RGCs impairs polarized organization and function of RGCs and the resultant neuronal layer formation in the developing cortex (E16). These APC-null defects were rescued by the codeletion of β-catenin and phenocopied by constitutive activation of β-catenin, indicating that β-catenin is a central effector of APC's role in cerebral cortical development. (A–E) RGCs were immunolabeled with radial glial-specific RC2 antibodies. APC loss completely disrupts RGC organization (cf. A and B), which was rescued by concomitant deletion of β-catenin (C). (D) β-Catenin deletion alone did not affect RGC organization, except for the occasional ectopias within the SVZ/IZ in β-catenin cKO cortices (asterisk). (E) Induction of active β-catenin in progenitors phenocopies the APC-null phenotype. (E′) Quantification of the number of polarized RC2+ basal fibers across unit width (100 µm) in the CP. (F–J) Proliferating cortical progenitors were pulse-labeled with BrdU. (J′) Quantification of the number of BrdU+ cells in the VZ. (K–O) Progenitors undergoing apoptosis were labeled with anti-cleaved caspase-3 antibodies. (O′) Quantification of changes in progenitor cell death. (P–T) IPs were immunolabeled with anti-Tbr2 antibodies. (T′) Quantification of Tbr2+ IPs. (U–Y) The emerging laminar organization of neurons was analyzed with layer-specific markers Ctip2 and Tbr1. (Y′) Changes in neuronal layer formation. Representative neuronal distribution plots from each genotype. Data shown are mean ± SEM. n = 3 for each genotype. (*) P < 0.05; (***) P < 0.001, one-way ANOVA with Tukey's multiple comparison test. Bars: A–E, 50 µm; F–J,K–O,P–T,U–Y, 60 µm.