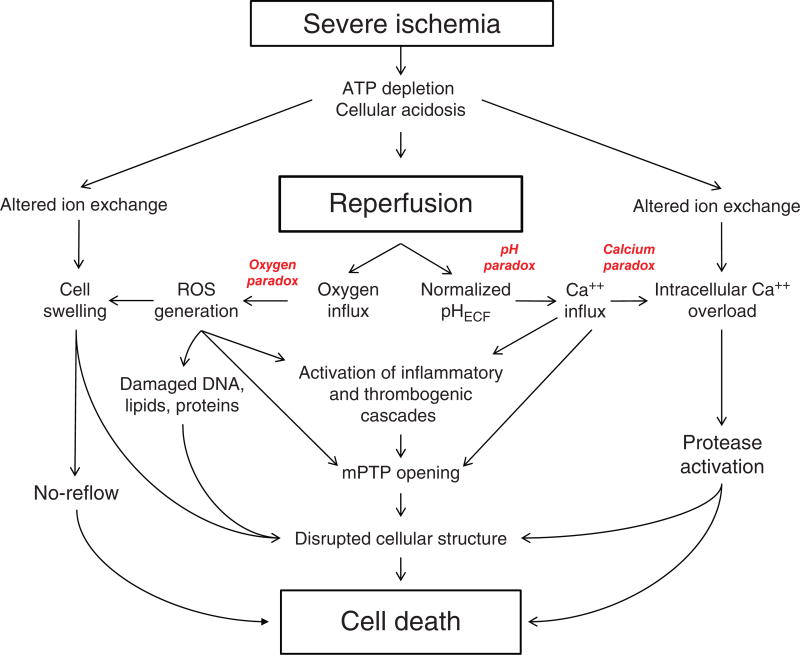
Figure 1.
Major pathologic events contributing to ischemia/reperfusion injury. When the blood supply is markedly reduced or absent, ischemic cells switch to anaerobic metabolism to provide ATP. However, this results in cellular acidosis and insufficient ATP production to meet metabolic demand. As a consequence, ATPases are inactivated, while active Ca2+ efflux and Ca2+ reuptake by the endoplasmic reticulum are markedly reduced, with the net effect of this abherent ion transport producing Ca2+ overload in the cell. In addition, xanthine dehydrogenase is converted to XO during ischemia (see Fig. 7), coincident with accumulation of hypoxanthine, one of the substrates required to drive its enzymatic activity. On reperfusion, the delivery of oxygen and substrates required for aerobic ATP generation is restored as is extracellular pH via washout of accumulated H+ (pH paradox). The latter event promotes additional Ca2+ influx (calcium paradox), while the influx of oxygen fuels XO-driven production of ROS (oxygen paradox) (see Fig. 7). ROS produced by this and other mechanisms can damage virtually every biomolecule found in cells, promote opening of mitochondrial PTPs, and activate inflammatory and thrombogenic cascades to exacerbate cell injury. The latter events are further amplified by release of danger signals (e.g., ATP) and other proinflammatory and thrombogenic mediators from damaged cells (see text for further explanation). The ensuing massive influx of immunocytes at previously ischemic sites contribute to cell injury via the NADPH oxidase-driven respiratory burst, release of hydrolytic enzymes, and production of MPO-derived hypochlorous acid and N-chloramines. The development of the capillary no-reflow phenomenon during reperfusion results in nutritive perfusion impairment by mechanisms outlined in Figure 11.