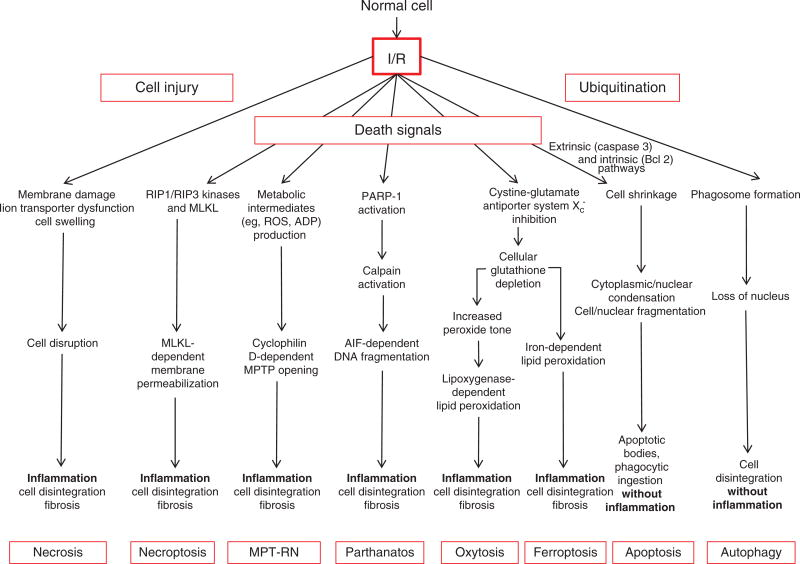
Figure 6.
Cell death modalities in ischemia/reperfusion (I/R). I/R-induced necrosis generally occurs as a result of dysfunctional ion transport mechanisms, which causes cells to swell and eventually burst, effects that are exacerbated by plasma membrane damage. Release of proinflammatory mediators and damaged biomolecules initiates the influx of inflammatory cells such as neutrophils, which disrupt the extracellular matrix and cause damage to parenchyal cells by release of cytotoxic oxidants and hydrolytic enzymes. Apopotosis is a regulated form of cell death that causes cell shrinkage and condensation of the cytosol and nucleus, which eventually form apoptotic bodies. Because they are surrounded by cell membranes, apoptotic bodies can be engulfed and digested by phagocytes without evoking an inflammatory response. Autophagy provides a mechanism to remove damaged or senescent protein aggregates and organelles by enclosing them in membrane-lined vesicles called proteasomes which fuse with lysosomes containing enzymes that degrade the ingested material, usually without evoking an inflammatory response. While normally performing this “housekeeping” function, autophagy may also provide cells with a survival mechanism to withstand the deleterious effects of ischemia, by generating amino acids and fatty acids for cell function. However, when uncontrolled, autophagy contributes to ischemic cell death. While necrosis was once believed to occur from non-specific trauma or injury as a result of I/R, it now appears that postischemic infarction may also be attributable to programmed events that require a dedicated molecular circuitry that has been termed programmed necrosis or necroptosis. Necroptosis is initiated by TNF-like cytokines that activate RIP kinases to mediate necrosis via increased production of reactive oxygen species and calcium overload, which in turn modulate the mitochondrial permeability transition pore (MPTP), leading to dissipation of the proton electrochemical gradient, with subsequent ATP depletion, further ROS production, and swelling and rupture of mitochondrial membranes. Recent genetic studies have suggested that the MPTP is predominantly involved in a second form of regulated necrosis that is designated MPT-RN that is critically dependent on cyclophilin D. Parthanotos can be distinguished from other forms of programmed cell death by its requirement for poly-ADP-ribose polymerase activation. Two newly described cell death modalities have been implicated in I/R, ferroptosis and oxytosis. Both involve inhibition of the cytine-glutamate antiporter Xc−, but differ in their modes of lipid peroxidation, being iron dependent and lipoxygenase dependent, respectively.