Abstract
Purpose of review:
Biosensors capable of measuring physiologic and kinetic parameters associated with disability are being applied to the study of people with multiple sclerosis (MS). We review the use of biosensors in people with MS with an emphasis on measuring/monitoring disability and understanding knowledge gaps between biosensor data and clinical care.
Recent findings:
Accelerometers are available to the public and may be able to help the clinician understand a patient's degree of disability. Further studies with wearable biosensors capable of measuring other physiologic features, such as vital signs, are needed and are likely to contribute to our understanding of MS.
Summary:
Wearable biosensors can improve our understanding of disability, response to treatment, and natural history of MS.
Multiple sclerosis (MS) is the most common demyelinating disease of the CNS and the most common cause of nontraumatic disability among young adults.1 People with MS (PwMS) experience fatigue, disordered sleep, gait difficulty, physical disability, and autonomic dysfunction that can adversely affect quality of life. A number of studies have investigated using biosensors to track exercise, physical activity, gait, balance, and disability in PwMS. A recent systematic review of 137 studies of remote activity monitoring in patients with neurologic disease listed 61 studies focusing on PwMS.2 This line of investigation holds potential for advancing our understanding of the natural history of MS in clinical practice.
Biosensors are devices capable of measuring a variety of physiologic and kinetic parameters. Wearable biosensors are now common in the community and accelerometers, in particular, have been used to study physical activity and energy expenditure in healthy adults. Actigraphy provides a noninvasive measurement of physical activity and can be used to quantify gross motor activity, footsteps, distance walked, sleep/wake patterns, and a number of other metrics. Modern devices are easily portable and can be worn on the wrist, ankle, or hip, and most detect physical activity through the use of accelerometers. Data are stored on a memory chip and can be transferred to a computer for analysis. Some devices are capable of measuring vital signs or other sophisticated metrics such as ambient light exposure, hip sway, or tremor.3
METHODS
We searched PubMed for MeSH terms such as “multiple sclerosis,” “accelerometry,” “biosensors,” “actigraphy,” “sleep,” “fatigue,” “gait,” and “autonomic dysfunction” in various combinations and reviewed selected articles based on perceived relevance to the topic.3 Selected studies identified in the references sections from articles under review were evaluated based on perceived relevance and included as appropriate.
Disability in MS
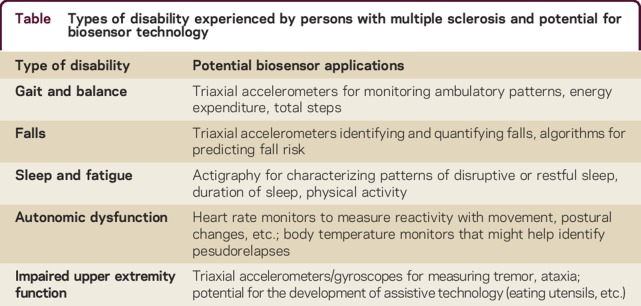
PwMS experience various types and degrees of disability, including motor impairment, vision loss, sensory loss, and bowel and bladder dysfunction (table). Cognitive dysfunction, fatigue, depression, and anxiety are other common nonphysical symptoms that can contribute to disability in MS. Autonomic dysfunction, sleep disturbances, and temperature sensitivity may contribute to symptoms and quality of life.4 Patient-reported outcomes can provide valuable information about disability and quality of life metrics, but are subject to response bias, reporting bias, and a number of other sources of error. Clinical metrics, such as the Expanded Disability Status Scale (EDSS) and the MS Functional Composite (MSFC), have been used to measure disability in MS.5 Such metrics are valuable tools in the clinical assessment of PwMS, but provide a limited snapshot of the patient's abilities. Our thesis is that combining the neurologic assessment with clinical metrics and continuous or daily monitoring with wearable biosensors may provide additional insights into the degree and variance of symptoms experienced by patients.
Table.
Types of disability experienced by persons with multiple sclerosis and potential for biosensor technology
Gait and balance
Walking impairment is an important measure of disability in MS. Almost 50% of PwMS report mobility impairment within the first month of diagnosis and over 90% report impaired mobility in the first 10 years.6 One survey found that 41% of PwMS reported difficulty walking, among whom 70% reported it was the most challenging aspect of having MS,7 and another found lower limb function was rated the most important of 13 bodily functions.8 The EDSS relies heavily upon walking ability, particularly over scores of 4.0,9 and the MSFC includes a measure of mobility (timed 25-foot walk) as well.10 The timed 25-foot walk and the 12-item MS walking scale are other useful measures of gait.5
Investigations in the laboratory setting, such as those above, have identified impaired temporospatial gait measures in people with recently diagnosed MS and low EDSS (0–2.5), including reduced speed, stride length, and prolonged double limb support, compared to healthy controls.11 Using an electronic walking mat with sensors arranged in a grid-like pattern in order to identify footfall contacts, researchers have characterized a number of temporospatial gait measures in PwMS.12 Those who were able to ambulate independently had slower walking velocity, took fewer steps per unit of time (decreased cadence), with shorter, wider steps, and spent more time spent in double support compared to age- and sex-matched controls. Similar findings were reproduced by other investigations and studies have linked data from such electronic walking mats with accelerometers that can be worn in the community.13
Wearable biosensors, accelerometers in particular, have great potential for further characterizing walking disability and movement measures of gait in PwMS. Triaxial accelerometers measure acceleration using a digital or piezoelectric sensor in 3 dimensions.14 The device quantifies the accelerations into activity counts over a period of time. One such device, the ActiGraph model 7164 uniaxial accelerometer (Health One Technology, Fort Walton Beach, FL), was among the best studied accelerometers in PwMS, until it was decommissioned and replaced by multiaxial accelerometers, such as the GT3X.14
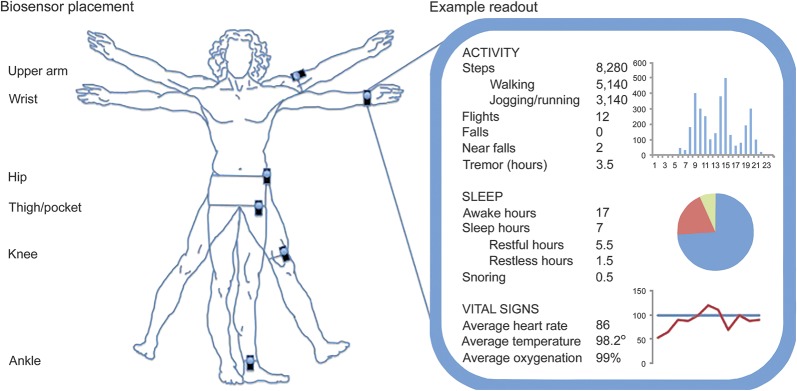
Accelerometers have recently become widely available to consumers. Studies investigating the accuracy and reliability of such devices in the laboratory setting are necessary to establish acceptability for clinical applications. Such studies have generally reported that most devices provide reasonably accurate measures of total energy expenditure.15 Smartphone applications are also likely relatively accurate.16 A study of PwMS with EDSS 1.0–5.0 compared commercially available sensors and smartphone applications during treadmill walking, and in keeping with previous studies demonstrated relative accuracy and precision, but decreasing precision at slower gait speeds.17 Monitor placement is important for data collection; some examples are provided in the figure. When using only one accelerometer, the thigh was the most accurate placement for static postures and movement.18 Whereas movement can be accurately classified with accelerometer placement on one or more sites (ankle, thigh, and waist), posture and transitions are best evaluated with 2 accelerometers, one on the waist and one on the thigh.
Figure. Example biosensor placement and readout.
A number of studies have used wearable biosensors to evaluate gait and balance in PwMS in the community. These have consistently shown that PwMS are less ambulatory than controls.14,19,20 The decreased amount of ambulation in the MS community is directly associated with disability, as evinced by higher EDSS scores in patients with lower average daily walking activity.20 A recent study of PwMS including both relapsing and progressive disease using commercially available and research accelerometers demonstrated moderate to strong correlation with other walking measures and importantly identified variability in real-world walking disability not apparent on EDSS.21 Such studies suggest a role for step-count monitoring as an exploratory outcome measure in clinical trials. Clinicians interested in employing accelerometers as part of their evaluation should bear in mind that these may be less accurate in patients with greater walking disability.22
Falls
Falls are common in PwMS and may be injurious to the patient.23 In addition to causing injury, studies have suggested that a fear of falling may cause patients to curtail their level of physical activity.24 Most studies have used patient-reported perceptions of the cause of their falls, which is subject to bias and misperception. One study using accelerometry demonstrated that PwMS with self-reported falls in the last year took significantly fewer steps than those who had not fallen.25 However, when controlling for disability, there was no significant difference between those who had fallen and those who had not.25 In patients with Parkinson disease, at least one actigraphic algorithm may help predict falls.26 Similar algorithms have not yet been applied to PwMS. Biosensors have the potential to detect and quantify falls and it may be possible to develop algorithms that could help predict falls in PwMS as well.
Autonomic dysfunction
Among the myriad autonomic functions, those commonly disrupted in PwMS include cardiovascular, thermoregulatory, gastrointestinal, and genitourinary/sexual functions.27,28 Symptoms such as heat and exercise intolerance, orthostasis, bowel and bladder dysfunction, and sexual dysfunction decrease quality of life for PwMS and can be disabling.
The prevalence of cardiovascular autonomic dysfunction in PwMS may be as high as 42%.29 In PwMS, there is reduced variation in resting heart rate30 and impaired withdrawal of tonic vagal activity in response to exercise, a finding that correlates with impaired walking capacity.31 One study reported a correlation between cardiovascular dysfunction and brainstem lesions in PwMS.32 Some authors have suggested that lesions in the brainstem are responsible for the cardiovascular autonomic dysfunction seen in PwMS,33 but this is uncertain.34 Decreased physical activity associated with MS may also affect the cardiovascular profile.35
The study of temperature and MS dates to back to the late 1800s, when Uhthoff made his observations.36 In the 1920s–1930s, hyperpyrexia was used therapeutically for PwMS. Since the 1950s, a number of investigations into the effects of temperature on PwMS have been undertaken.36 Recently, some studies have suggested variation in MS disease severity with elevated outdoor temperatures, although this is an area of debate and seasonal variation in MS disease activity may be influenced by a number of factors.37 Increased outdoor temperatures were associated with worse cognitive function in PwMS,38 but a more recent review did not find such evidence.39 Some studies have supported seasonal variation in disease activity; for example, one cohort study of 44 untreated PwMS found that new T2 lesions were 2–3 times more common in March–August than during the rest of the year and correlated with regional climate data, suggesting more disease activity during the hotter months.40
A recent cross-sectional study including 50 patients with relapsing-remitting MS (RRMS), 40 healthy controls, and 22 patients with secondary progressive MS (SPMS) reported that body temperature was higher in patients with RRMS (37.04 ± 0.27°C) compared to healthy controls (36.83 ± 0.33; p = 0.009) and patients with SPMS (36.75 ± 0.39°C; p = 0.001).41 Higher body temperature correlated with worse fatigue but not cognitive fatigue. The same group reported similar findings in another cohort of patients with RRMS.42
Wearable biosensors, including many commercially available models, are capable of measuring vital signs, especially heart rate, but have only just begun to be applied to the study of PwMS. Such investigations may help further describe autonomic dysfunction in the disease. Ambulatory thermosensors capable of correlating heat-related disability are one possible application. Core temperature monitors, such as an ingestible thermosensor capsule, might be employed to this end and studies have begun to emerge utilizing portable core temperature monitoring systems.43
Sleep and fatigue
PwMS experience a variety of sleep disorders that can affect quality of life.44 Sleep disturbances are more common among PwMS than in the general population; for example, insomnia occurs in over 40% of PwMS, compared to 10%–15% in the general population. Symptoms of MS may also cause secondary sleep disruptions related to neurogenic bladder, or stiffness and pain related to spasticity.
Whereas many studies have investigated the use of actigraphy in patients with primary sleep disorders, Parkinson disease, and other psychiatric and neurologic disorders, few studies have examined the application to the study of sleep in MS. There is a correlation between disturbed sleep, abnormal sleep cycles, and fatigue.45 MS disease-modifying therapeutics may contribute to sleep disorders in MS. One study monitored 44 patients with mild RRMS via actigraphy for at least 7 days.46 The authors found an average decrease in sleep efficiency of 5% on two-thirds of nights following interferon injections and actigraphy correlated with daily sleep ratings. Patients on glatiramer acetate also had reduced sleep efficiency.46 Further studies using patient-reported data including fatigue and sleep logs coupled with objective findings from actigraphy may help elucidate what underlying relationship, if any, exists between disease-modifying therapy and sleep disturbance in MS.
Fatigue is a common symptom affecting PwMS. The relationship between sleep and fatigue in MS is complex and has not yet been fully elucidated and there is opportunity for the application of biosensors in this area. For example, one study monitored 6 patients with fatigue and untreated RRMS for 48 hours using polysomnography and body core temperature (via rectal probe) followed by the Multiple Sleep Latency Test.47 The authors found normal sleep-wake and body core temperature rhythmicity in this small sample. Further studies in this area are warranted.
Other investigators have focused on overall physical activity and its relationship with fatigue in MS. Physical inactivity is prevalent in PwMS48 and increases the risk of cardiovascular disease associated with disability progression.49 Data from both cross-sectional and longitudinal studies demonstrate bidirectional interactions among fatigue, depression, and decreased physical activity.50 People with depression are less physically active, and actigraphy may serve as an additional monitoring measure for PwMS who are depressed.51 Increased physical activity correlates with improved disability status.52 Some studies suggest beneficial effects of exercise for PwMS including improved walking disability,53 fatigue,54 and quality of life,55 although a minority of the literature is based on randomized controlled trials.56
Limitations
There are a number of factors that may limit the application and use of wearable biosensors in the study of MS. Patient adherence is one potential barrier to data collection and patients may be reticent to adhere to the protocol out of inconvenience or concern about the appearance of the sensor; however, at least one study has suggested that this is not likely to be a major impediment.57 Improper sensor placement (such as in the pocket rather than on the hip) is a potential source of data contamination. The Hawthorne effect, wherein study participants change their behavior while under observation, is another potential problem.
The ongoing technological evolution of accelerometers creates a challenge for researchers as different models do not necessarily agree when directly compared.58 For example, one study comparing models in PwMS and healthy controls noted a 7% difference in output between the ActiGraph 7164 and the GT3X accelerometers, which was likely explained by differences in slow walking activity capture.14 Thus comparison studies are important when evaluating actigraphy results generated by different models.59 Multiple studies have generally reported good agreement between current models.60,61 One recent study compared commercially available and research actigraphs and reported that the commercially available monitors overall provided reasonably accurate estimates of total energy expenditure, but larger error was noted for individual activities, particularly resistance exercise.15 However, a recent comparison of several commercially available smartphone accelerometer applications found substantial variability in their precision and accuracy.17
Future investigations
Wearable biosensor technology has the potential to deepen our understanding of disability associated with MS, but also to improve our understanding of MS natural history and treatment response. Natural history studies incorporating wearable biosensors might investigate predictors of patterns of disease, clinical course, relapses, and disability and should help to further elucidate autonomic dysfunction in PwMS. Biosensor data might be correlated with quality of life metrics and are beginning to be applied to the clinical setting, and clinicians might consider prescribing activity and fitness goals. In addition, biosensor-generated data can complement biological and radiologic markers for novel clinical trial outcome metrics. Such efforts could result in a new generation of knowledge regarding the application of biosensors for monitoring PwMS.
Take-home points
Wearable biosensors are capable of measuring a variety of physiologic and kinetic measures
Wearable biosensors can be used for monitoring disability in people with MS, most readily physical activity, sleep metrics, and heart rate
Accelerometers such as those found in smartphones are relatively accurate, but lose accuracy at slower walking speeds/higher EDSS
Wearable biosensors are being incorporated into natural history studies and have potential value for generating novel clinical trial outcome measures
AUTHOR CONTRIBUTIONS
M.J. Bradshaw: concept design, literature review, manuscript composition, drafting, revision. S. Farrow: literature review, manuscript preparation, revision. R. Motl: literature review, manuscript preparation, revision. T. Chitnis: concept design, manuscript editing/revision.
STUDY FUNDING
No targeting funding reported.
DISCLOSURES
M.J. Bradshaw and S. Farrow report no disclosures. R.W. Motl served as an Associate Editor for Neurorehabilitation and Neural Repair; serves as a consultant for Biogen and Acorda Therapeutics; serves on the speakers' bureau for EMD Serono; and receives research support from Biogen, Acorda, Sun Health Technologies, and the National MS Society. T. Chitnis has served on clinical trial advisory boards for Novartis and Genzyme-Sanofi; has served as a consultant for Biogen-Idec, Novartis, Genzyme-Sanofi, Teva Neurosciences, and Genentech-Roche; receives research support from EMD Serono, Novartis, Biogen, and Verily in the form of Independent Investigator Awards; and receives research support from NIH, National Multiple Sclerosis Society, the Peabody Foundation, the Consortium for MS Centers, and the Guthy-Jackson Charitable Foundation. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.

REFERENCES
- 1.Pugliatti M, Rosati G, Carton H, et al. . The epidemiology of multiple sclerosis in Europe. Eur J Neurol 2006;13:700–722. [DOI] [PubMed] [Google Scholar]
- 2.Block VA, Pitsch E, Tahir P, Cree BA, Allen DD, Gelfand JM. Remote physical activity monitoring in neurological disease: a systematic review. PLoS One 2016;11:e0154335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motl RW, Sandroff BM, Sosnoff JJ. Commercially available accelerometry as an ecologically valid measure of ambulation in individuals with multiple sclerosis. Expert Review Neurother 2012;12:1079–1088. [DOI] [PubMed] [Google Scholar]
- 4.Veauthier C, Paul F. Sleep disorders in multiple sclerosis and their relationship to fatigue. Sleep Med 2014;15:5–14. [DOI] [PubMed] [Google Scholar]
- 5.Motl RW, Cohen JA, Benedict R, et al. . Validity of the timed 25-foot walk as an ambulatory performance outcome measure for multiple sclerosis. Mult Scler 2017;23:704–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Asch P. Impact of mobility impairment in multiple sclerosis 2: patients' perspectives. Eur Neurol Rev 2011;6:115–120. [Google Scholar]
- 7.Larocca NG. Impact of walking impairment in multiple sclerosis: perspectives of patients and care partners. Patient 2011;4:189–201. [DOI] [PubMed] [Google Scholar]
- 8.Heesen C, Bohm J, Reich C, Kasper J, Goebel M, Gold SM. Patient perception of bodily functions in multiple sclerosis: gait and visual function are the most valuable. Mult Scler 2008;14:988–991. [DOI] [PubMed] [Google Scholar]
- 9.Twork S, Wiesmeth S, Spindler M, et al. . Disability status and quality of life in multiple sclerosis: non-linearity of the Expanded Disability Status Scale (EDSS). Health Qual Life Outcomes 2010;8:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer JS, Rudick RA, Cutter GR, Reingold SC. The Multiple Sclerosis Functional Composite Measure (MSFC): an integrated approach to MS clinical outcome assessment: National MS Society Clinical Outcomes Assessment Task Force. Mult Scler 1999;5:244–250. [DOI] [PubMed] [Google Scholar]
- 11.Martin CL, Phillips BA, Kilpatrick TJ, et al. . Gait and balance impairment in early multiple sclerosis in the absence of clinical disability. Mult Scler 2006;12:620–628. [DOI] [PubMed] [Google Scholar]
- 12.Givon U, Zeilig G, Achiron A. Gait analysis in multiple sclerosis: characterization of temporal-spatial parameters using GAITRite functional ambulation system. Gait Posture 2009;29:138–142. [DOI] [PubMed] [Google Scholar]
- 13.Motl RW, Pilutti L, Sandroff BM, Dlugonski D, Sosnoff JJ, Pula JH. Accelerometry as a measure of walking behavior in multiple sclerosis. Acta Neurol Scand 2013;127:384–390. [DOI] [PubMed] [Google Scholar]
- 14.Sandroff BM, Motl RW. Comparison of ActiGraph activity monitors in persons with multiple sclerosis and controls. Disabil Rehabil 2013;35:725–731. [DOI] [PubMed] [Google Scholar]
- 15.Bai Y, Welk GJ, Nam YH, et al. . Comparison of consumer and research monitors under semistructured settings. Med Sci Sports Exerc 2016;48:151–158. [DOI] [PubMed] [Google Scholar]
- 16.Case MA, Burwick HA, Volpp KG, Patel MS. Accuracy of smartphone applications and wearable devices for tracking physical activity data. JAMA 2015;313:625–626. [DOI] [PubMed] [Google Scholar]
- 17.Balto JM, Kinnett-Hopkins DL, MOtl RW. Accuracy and precision of smartphone applications and commercially available motion sensors in multiple sclerosis. Translational Clin 2016;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lugade V, Fortune E, Morrow M, Kaufman K. Validity of using tri-axial accelerometers to measure human movement: part I: posture and movement detection. Med Eng Phys 2014;36:169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weikert M, Suh Y, Lane A, et al. . Accelerometry is associated with walking mobility, not physical activity, in persons with multiple sclerosis. Med Eng Phys 2012;34:590–597. [DOI] [PubMed] [Google Scholar]
- 20.Gijbels D, Alders G, Van Hoof E, et al. . Predicting habitual walking performance in multiple sclerosis: relevance of capacity and self-report measures. Mult Scler 2010;16:618–626. [DOI] [PubMed] [Google Scholar]
- 21.Block VJ, Lizee A, Crabtree-Hartman E, et al. . Continuous daily assessment of multiple sclerosis disability using remote step count monitoring. J Neurol 2017;264:316–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandroff BM, Riskin BJ, Agiovlasitis S, Motl RW. Accelerometer cut-points derived during over-ground walking in persons with mild, moderate, and severe multiple sclerosis. J Neurol Sci 2014;340:50–57. [DOI] [PubMed] [Google Scholar]
- 23.Matsuda PN, Shumway-Cook A, Bamer AM, Johnson SL, Amtmann D, Kraft GH. Falls in multiple sclerosis. PM R 2011;3:624–632; quiz 632. [DOI] [PubMed] [Google Scholar]
- 24.Peterson EW, Cho CC, Finlayson ML. Fear of falling and associated activity curtailment among middle aged and older adults with multiple sclerosis. Mult Scler 2007;13:1168–1175. [DOI] [PubMed] [Google Scholar]
- 25.Sosnoff JJ, Sandroff BM, Pula JH, Morrison SM, Motl RW. Falls and physical activity in persons with multiple sclerosis. Mult Scler Int 2012;2012:315620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iluz T, Gazit E, Herman T, et al. . Automated detection of missteps during community ambulation in patients with Parkinson's disease: a new approach for quantifying fall risk in the community setting. J Neuroeng Rehabil 2014;11:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Racosta JM, Kimpinski K. Autonomic dysfunction, immune regulation, and multiple sclerosis. Clin Auton Res 2016;26:23–31. [DOI] [PubMed] [Google Scholar]
- 28.Habek M, Crnosija L, Lovric M, Junakovic A, Krbot Skoric M, Adamec I. Sympathetic cardiovascular and sudomotor functions are frequently affected in early multiple sclerosis. Clin Auton Res 2016;26:385–393. [DOI] [PubMed] [Google Scholar]
- 29.Racosta JM, Sposato LA, Morrow SA, Cipriano L, Kimpinski K, Kremenchutzky M. Cardiovascular autonomic dysfunction in multiple sclerosis: a meta-analysis. Mult Scler Relat Disord 2015;4:104–111. [DOI] [PubMed] [Google Scholar]
- 30.Neubauer B, Gundersen HJ. Analysis of heart rate variations in patients with multiple sclerosis: a simple measure of autonomic nervous disturbances using an ordinary ECG. J Neurol Neurosurg Psychiatry 1978;41:417–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hansen D, Wens I, Dendale P, Eijnde BO. Exercise-onset heart rate increase is slowed in multiple sclerosis patients: does a disturbed cardiac autonomic control affect exercise tolerance? NeuroRehabilitation 2013;33:139–146. [DOI] [PubMed] [Google Scholar]
- 32.Acevedo AR, Nava C, Arriada N, Violante A, Corona T. Cardiovascular dysfunction in multiple sclerosis. Acta Neurol Scand 2000;101:85–88. [DOI] [PubMed] [Google Scholar]
- 33.Vita G, Fazio MC, Milone S, Blandino A, Salvi L, Messina C. Cardiovascular autonomic dysfunction in multiple sclerosis is likely related to brainstem lesions. J Neurol Sci 1993;120:82–86. [DOI] [PubMed] [Google Scholar]
- 34.Videira G, Castro P, Vieira B, et al. . Autonomic dysfunction in multiple sclerosis is better detected by heart rate variability and is not correlated with central autonomic network damage. J Neurol Sci 2016;367:133–137. [DOI] [PubMed] [Google Scholar]
- 35.Ranadive SM, Yan H, Weikert M, et al. . Vascular dysfunction and physical activity in multiple sclerosis. Med Sci Sports Exerc 2012;44:238–243. [DOI] [PubMed] [Google Scholar]
- 36.Guthrie TC, Nelson DA. Influence of temperature changes on multiple sclerosis: critical review of mechanisms and research potential. J Neurol Sci 1995;129:1–8. [DOI] [PubMed] [Google Scholar]
- 37.Watad A, Azrielant S, Soriano A, Bracco D, Abu Much A, Amital H. Association between seasonal factors and multiple sclerosis. Eur J Epidemiol 2016;31:1081–1089. [DOI] [PubMed] [Google Scholar]
- 38.Leavitt VM, Sumowski JF, Chiaravalloti N, Deluca J. Warmer outdoor temperature is associated with worse cognitive status in multiple sclerosis. Neurology 2012;78:964–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberg BL, Bruce JM. Reconsidering outdoor temperature and cognition in multiple sclerosis. Mult Scler 2016;22:694–697. [DOI] [PubMed] [Google Scholar]
- 40.Meier DS, Balashov KE, Healy B, Weiner HL, Guttmann CR. Seasonal prevalence of MS disease activity. Neurology 2010;75:799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sumowski JF, Leavitt VM. Body temperature is elevated and linked to fatigue in relapsing-remitting multiple sclerosis, even without heat exposure. Arch Phys Med Rehabil 2014;95:1298–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leavitt VM, De Meo E, Riccitelli G, et al. . Elevated body temperature is linked to fatigue in an Italian sample of relapsing-remitting multiple sclerosis patients. J Neurol 2015;262:2440–2442. [DOI] [PubMed] [Google Scholar]
- 43.Sandroff BM, Motl RW, Davis SL. Effects of vigorous walking exercise on core body temperature and inhibitory control in thermosensitive persons with multiple sclerosis. Neurodegener Dis Manag 2016;6:13–21. [DOI] [PubMed] [Google Scholar]
- 44.Braley TJ, Boudreau EA. Sleep disorders in multiple sclerosis. Curr Neurol Neurosci Reports 2016;16:50. [DOI] [PubMed] [Google Scholar]
- 45.Attarian HP, Brown KM, Duntley SP, Carter JD, Cross AH. The relationship of sleep disturbances and fatigue in multiple sclerosis. Arch Neurol 2004;61:525–528. [DOI] [PubMed] [Google Scholar]
- 46.Mendozzi L, Tronci F, Garegnani M, Pugnetti L. Sleep disturbance and fatigue in mild relapsing remitting multiple sclerosis patients on chronic immunomodulant therapy: an actigraphic study. Mult Scler 2010;16:238–247. [DOI] [PubMed] [Google Scholar]
- 47.Vetrugno R, Stecchi S, Scandellari C, et al. . Sleep–wake and body core temperature rhythms in multiple sclerosis with fatigue. Clin Neurophysiol 2007;118:228–234. [DOI] [PubMed] [Google Scholar]
- 48.Motl RW, McAuley E, Snook EM. Physical activity and multiple sclerosis: a meta-analysis. Mult Scler 2005;11:459–463. [DOI] [PubMed] [Google Scholar]
- 49.Marrie RA, Rudick R, Horwitz R, et al. . Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology 2010;74:1041–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Motl RW, Suh Y, Weikert M, Dlugonski D, Balantrapu S, Sandroff B. Fatigue, depression, and physical activity in relapsing-remitting multiple sclerosis: results from a prospective, 18-month study. Mult Scler Relat Disord 2012;1:43–48. [DOI] [PubMed] [Google Scholar]
- 51.Schuch F, Vancampfort D, Firth J, et al. . Physical activity and sedentary behavior in people with major depressive disorder: a systematic review and meta-analysis. J Affect Disord 2017;210:139–150. [DOI] [PubMed] [Google Scholar]
- 52.Motl RW, Snook EM, Wynn DR, Vollmer T. Physical activity correlates with neurological impairment and disability in multiple sclerosis. J Nerv Ment Dis 2008;196:492–495. [DOI] [PubMed] [Google Scholar]
- 53.Pearson M, Dieberg G, Smart N. Exercise as a therapy for improvement of walking ability in adults with multiple sclerosis: a meta-analysis. Arch Phys Med Rehabil 2015;96:1339–1348. [DOI] [PubMed] [Google Scholar]
- 54.Pilutti LA, Greenlee TA, Motl RW, Nickrent MS, Petruzzello SJ. Effects of exercise training on fatigue in multiple sclerosis: a meta-analysis. Psychosom Med 2013;75:575–580. [DOI] [PubMed] [Google Scholar]
- 55.Motl RW, Gosney JL. Effect of exercise training on quality of life in multiple sclerosis: a meta-analysis. Mult Scler 2008;14:129–135. [DOI] [PubMed] [Google Scholar]
- 56.Motl RW, Pilutti LA. The benefits of exercise training in multiple sclerosis. Nat Rev Neurol 2012;8:487–497. [DOI] [PubMed] [Google Scholar]
- 57.McIninch J, Datta S, DasMahapatra P, et al. . Remote tracking of walking activity in MS patients in a real-world setting (P3.209). Neurology 2015;84. [Google Scholar]
- 58.Stalesen J, Vik FN, Hansen BH, Berntsen S. Comparison of three activity monitors for estimating sedentary time among children. BMC Sports Sci Med Rehabil 2016;8:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paul DR, Kramer M, Moshfegh AJ, Baer DJ, Rumpler WV. Comparison of two different physical activity monitors. BMC Med Res Methodol 2007;7:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robusto KM, Trost SG. Comparison of three generations of ActiGraph activity monitors in children and adolescents. J Sports Sci 2012;30:1429–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Unick JL, Bond DS, Jakicic JM, et al. . Comparison of two objective monitors for assessing physical activity and sedentary behaviors in bariatric surgery patients. Obes Surg 2012;22:347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]