Abstract
Introduction
Oncogenic Kras mutations are important drivers of lung cancer development and metastasis. They are known to activate numerous cellular signaling pathways implicated in enhanced proliferation, survival, tumorigenicity and motility during malignant progression.
Objectives
Most previous studies of Kras in cancer have focused on the comparison of cell states in the absence or presence of oncogenic Kras mutations. Here we show that differential expression of the constitutively active mutation KrasV12 has profound effects on cell morphology and motility that drive metastatic processes.
Methods
The study relies on lung cancer cell transformation models, patient-derived lung cancer cell lines, and human lung tumor sections combined with molecular biology techniques, live-cell imaging and staining methods.
Results
Our analysis shows two cell functional states driven by KrasV12 protein levels: a non-motile state associated with high KrasV12 levels and tumorigenicity, and a motile state associated with low KrasV12 levels and cell dissemination. Conversion between the states is conferred by differential activation of a mechano-sensitive double-negative feedback between KrasV12/ERK/Myosin II and matrix-adhesion signaling. KrasV12 expression levels change upon cues such as hypoxia and integrin-mediated cell-matrix adhesion, rendering KrasV12 levels an integrator of micro-environmental signals that translate into cellular function. By live cell imaging of tumor models we observe shedding of mixed high and low KrasV12 expressers forming multi-functional collectives with potentially optimal metastatic properties composed of a highly mobile and a highly tumorigenic unit.
Discussion
Together these data highlight previously unappreciated roles for the quantitative effects of expression level variation of oncogenic signaling molecules in conferring fundamental alterations in cell function regulation required for cancer progression.
Keywords: Cancer, Hypoxia, Kras, Motility, Adhesion
Introduction
Kras belongs to the GTPase family of molecular switches that initiate several signaling cascades (Boettner and Van Aelst, 2002) such as the MAPK (mitogen-activated protein kinases) and the PI3-kinase pathways in response to growth factor receptor stimulation. Activation of these pathways leads to changes in gene transcription regulation (Roberts and Stinchcombe, 2013) as well as shifts in cytoskeletal dynamics (Malliri and Collard, 2003). In particular, extracellular signal-regulated kinase 1/2 (ERK) as part of the MAPK pathway can regulate Actin dynamics (Mendoza et al., 2011b, Mendoza et al., 2011a, Mendoza et al., 2015) or Myosin II activity (Klemke et al., 1997, Stupack et al., 2000) causing changes in cell motility and invasion. In many cancers Kras is associated with gain-of-function mutations (GOF) (Prior et al., 2012). Such mutations cause enhanced proliferation and survival leading to malignant tumorigenicity. They also contribute to a higher risk of metastasis (Pylayeva-Gupta et al., 2011). In the particular case of lung cancer, GOF mutations are generally related to a constitutively active form of the Kras protein (Scheffzek et al., 1997), which is thought to dramatically elevate the activation of downstream pathways independent of growth factor signaling (Schubbert et al., 2007). Among these mutations the glycine to valine mutation at codon 12 (KrasV12) leads to enhanced metastatic potential and therefore is considered to be among the most lethal (Ihle et al., 2012).
Aberrant cell motility and dissemination is one characteristic of metastatic cancer cells and represents the most challenging problem in clinical cancer research. During this process cancer cells need to detach from the primary tumor, move through different extracellular environments and spread to distant sites. In order to metastasize efficiently, cancer cells switch between various modes of motility e.g. from amoeboid to mesenchymal or in some instances migrate collectively (Sanz-Moreno et al., 2008, Friedl, 2004, Friedl et al., 2012). Such morpho-dynamic plasticity provides the cancer cells with an enhanced capability of adaptation to different external environments (Sahai, 2005). This malignant cellular behavior influences the response to standard cancer therapies and therefore has strong clinical implications (Aparicio et al., 2015).
In addition to oncogenic changes and cellular plasticity, tumor hypoxia is also correlated with poor prognosis in lung cancer (Vaupel and Mayer, 2007). Hypoxia promotes stem-like characteristics of cancer cells, tumor growth, and dissemination (Axelson et al., 2005, Harris, 2002, Keith and Simon, 2007). It was also shown that hypoxia induces extracellular matrix (ECM) remodeling and thus affects the composition and organization of the tumor microenvironment (Gilkes et al., 2014). Biophysical and biological properties of the ECM directly regulate cellular motility and metastatic behavior (Lo et al., 2000, Levental et al., 2009), for example mediated by focal adhesion kinase (FAK) activation, which promotes tumor progression, metastasis, motility and drug resistance (Sulzmaier et al., 2014, Hirata et al., 2015). All together these previous studies indicate the importance of external signals in the regulation of cellular response and behavior.
In this study, we analyzed the effect of hypoxia and ECM stiffness on KrasV12 protein levels in lung cancer morphogenesis. We found that KrasV12 protein levels are substantially up-regulated upon long-term hypoxia. Such changes in KrasV12 protein concentration control a morpho-dynamic switch to sphere-like, tumorigenic and immobile cells. Upon adhesion initiation, KrasV12 levels are down-regulated, which in turn induces adoption of a spreading and highly mobile phenotype. The switch between phenotypes is conferred by differential activation of a mechano-sensitive double-negative feedback between KrasV12/ERK/Myosin II and matrix-adhesion signaling. Additionally, we found that clusters of cells with different KrasV12 levels and thus different motile function can disseminate together as a multi-functional unit. With this we established that variations in the expression levels of a mutated oncogene, here KrasV12, rather than harboring the mutation itself, regulate profound switches in cellular function and behavior.
Methods
Cell lines, cell culture, clonal separation, sphere and collagen assay
Human bronchial epithelial cells immortalized with Cdk4 and hTERT expression (HBEC) or immortalized HBEC transformed with p53 knock-down, KrasV12 and cMyc expression (HBECp53-cMyc-Kras) (Sato et al., 2013) were cultured in keratinocyte serum-free medium (KSFM, Gibco) supplemented with 50 mg/ml of bovine pituitary extract (Gibco), 5 ng/ml of EGF (Gibco) and 1% Anti-Anti (Gibco) in a humidified incubator at 37°C, 5% CO2 and 21% O2 or at 2% or 1% O2 in special hypoxia incubators (Nuaire) where O2 is exchanged with N2. For analysis of the effect of hypoxia (1–2% O2), cell lines were cultured in long-term hypoxia which was defined as >4weeks. If not otherwise indicated, all experiments and cell culture was performed in 2% O2. Only for normoxia (21% O2) and hypoxia comparison, cells were cultured in 21% O2. To induce sphere formation, HBECp53-cMyc-Kras or clones were cultured in low attachment dishes (Corning) for 10–11 days before the experiments. For re-attachment, spheres were dispersed, seeded into plastic dishes and cultured for 10 days before imaging and lysis. For adherent and sphere-like cell population comparison in H2887 and HBECp53-cMyc-Kras, spheres that were mostly floating in the media were collected by spinning down the supernatant from cell culture in regular plastic dishes. Remaining attached cells from the same dish were lysed separately. Single clones were isolated from HBECp53-cMyc-Kras by diluting the cell counts down to ~90 single cells total and spreading these cells throughout a 96-well plate. Wells with more than one clonal colony were dismissed. Clones were sub-cultured after reaching ~70% confluence and categorized as low or high KrasV12 clone based on their KrasV12 expression level analyzed by western blot. For the migration assay in collagen, bovine Collagen I (Advanced BioMatrix) was neutralized to pH: 7 and diluted 1:3.4 in KFSM. Afterwards, the mix was transferred into glass bottom dishes (LAB-TEK) and solidified for 1.5 h at 37°C which created a layer of ~720 μm thickness. HBECp53-cMyc-Kras were seeded on top of the matrix in 1:3 diluted KSFM/PBS. Cells were imaged 2 days after seeding. The patient-derived lung cancer cell line H2887 and HCC4017 were provided by Prof. Minna (UT Southwestern Medical Center). These cells were cultured in RPMI 1640 medium (Gibco) supplemented with 5% cosmic calf serum (HyClone) and 1% Anti-Anti in a humidified incubator at 37°C, 5% CO2 and 21%, 2% or 1% O2. All cell lines were tested negative for Mycoplasma by using the Mycoplasma detection kit (Genlantis).
Cloning, virus production, infection and cell sorting
pLVX-GFP-KrasV12 vector was constructed by cloning a SpeI/NotI-digested KrasV12 fragments from pLenti-KrasV12-vector (Vikis et al., 2007) into pLVX-IRES-Neomycin vector (Clontech). EcoRI/SpeI-digested GFP insert was ligated into the pLVX-IRES-Neomycin vector as N-terminal fusion protein. The virus was produced to the manufacturer’s specifications. After collection of the virus, HBECp53-cMyc-Kras cells were spin infected at 3000 rpm for 1 h at room temperature with the addition of 2 ug/ml Polybrene and briefly cultured in 21% O2 to overcome hypoxia-induced reduction of infection efficiency. Transduced cells were then further cultured in 2% O2 and selected with G418 (800 μg/ml, Gibco). Additionally, selected cells were sorted for GFP expression using a FACS Aria II sorter (BD Bioscience).
To down-regulate KrasV12, pGIPZ shRNA against wild-type and mutated Kras (Oligo ID.: V2LHS_275818) from Dharmacon and a non-silencing shRNA (Dharmacon) was used as control. For virus production, HEK293FT cells were infected with 500 ng of shRNA against Kras, 200 ng of pMD2G and 300 ng psPAX2 using the transfection reagent Effectene (Qiagen) which was used according to manufacturer’s protocol. Virus was collected starting 24 h after transfection in a 4:1 ratio of Dulbecco’s modified Eagle’s medium to Medium 199 containing 10% cosmic calf serum. HBECp53-cMyc-Kras were treated with virus-containing media supplemented with 2 ug/ml Polybrene initially 1x for 5.5 h and 2x repetitively overnight. Afterwards, infected cells were selected with 3x repetitive overnight cycles using 10 ug/ml Puromycin diluted in KSFM. Subsequently, protein lysates were prepared and analyzed by western blot for total (wild-type and mutated) Kras down-regulation. Cells were imaged to determine change in morphology 6 days after selection.
Cell perturbations
High KrasV12 clones were treated with an initial dose of 20 nM of SCH772984 (Morris et al., 2013) (ChemieTek) for 48–68 h followed by 50 nM SCH772984 treatment for 24–48 h or with equivalent concentration of DMSO diluted in KSFM as control. After this treatment, samples were collected for western blot analysis. Live-cell imaging was started after increase to 50 nM SCH772984.
High KrasV12 clones were incubated with 500 nM blebbistatin (Sigma) or with equal concentration of DMSO in KFSM as control for 48 h. During this time, treatment was renewed every 24 h before samples were lysed for western blot analysis or cells were imaged documenting morphology changes. Live-cell imaging for migration analysis was started 43 h after treatment.
Low KrasV12 clones were treated with 50 μM FAK inhibitor 14 (Golubovskaya et al., 2008) (Tocris) or with equivalent concentration of DMSO diluted in KSFM as control for 48 and 72 h. After initial 24 h, treatment was renewed. Images were taken after 48 h and samples for western blot analysis were collected 48 and 72 h after treatment.
Low KrasV12 clones were cultured in 1 or 2 nM of CalyculinA (Sigma) or in equivalent concentration of DMSO diluted in KFSM as control for 12–48 h. Images were taken 48 h after treatment. Samples for western blot analysis were lysed 12–48 h after treatment.
Elastically supported surfaces (ESS) with a stiffness of 28, 15 and 1.5 kPa were purchased from Ibidi. Before usage, these surfaces were washed with PBS. ESS as well as plastic dishes were coated with 10 ug/ml BSA (bovine serum albumin, Sigma) or with 0.5% Gelatin for H2887 culture for 1 h at 37°C. Afterwards, low KrasV12 cells or H2887 were seeded in the center of the dishes and let adhere for minimally 3 h before filling up with medium. Live-cell imaging of low KrasV12 samples was started 24 h, samples for western blot analysis were collected 24–48 h and images were taken 48 h after seeding. H2887 were cultured 24 h before images were taken and samples were lysed for western blot analysis. Morphologies were analyzed by categorizing the cells as adherent or sphere-like using phase contrast images. Percentages of adherent versus sphere-like cells were quantified and the mean plus standard deviation for six images per condition was calculated.
Disc-patterned, ready-to-coat CYTOOchips (CYTOO) were coated with 5, 20, 100 or 200 μg/ml bovine Collagen I (Advanced BioMatrix) using a 4 well CYTOOchamber (CYTOO) for 2 h at room temperature. Afterwards, the wells were washed 4 times with PBS and high or low KrasV12 cells were seeded in a density of 50,000 cells per ml in a volume of 100 μl and let spread for 1 h at 37°C in 2% O2. Cell morphologies were assessed by phase contrast imaging using a CO2, O2 and heat controlled microscope system. Different areas per condition were imaged and each individual experiment was repeated 3 times. Cell morphologies were quantified as described above. Here, 11 to 16 data points were analyzed.
Microscopy and live-cell imaging
All live-cell (except the 3D collagen migration assay), immunofluorescence (IF) as well as the soft-agar z-stack imaging were performed on an inverted phase contrast and fluorescence Eclipse Ti microscope (Nikon) equipped with perfect focus, motorized stage for parallel data acquisition, CO2 control, heated and humidified air for long term live-cell imaging, O2 control for hypoxia imaging, a Zyla sCMOS camera, SOLA solid state white-light excitation system and a motorized filter turret with filters for DAPI, FITC and Texas Red. Nikon Elements was used as image acquisition software. All live-cell imaging was performed in 2% O2 cultivation. For imaging of soft-agar assay, a 4x Plan Fluor objective, for Myosin II IF a 60x Plan Apo VC oil objective and for live-cell imaging and for Kras IF the 10x or 20x Plan Fluor objectives were used.
A standard phase contrast cell culture microscope Eclipse TS100 (Nikon) using a 4x Plan Fluor or 10x Ph1 objective equipped with a fluorescence lamp (Lumencor), FITC filter sets, a camera (IMAGINGSOURCE) and the IC Capture software (IMAGINGSOURCE) was used to document cell morphologies as well as an inverted Axiovert 200M microscope (Zeiss) equipped with a AxioCam MRm camera (Zeiss) and Axiovision software (Zeiss) using a 10x or 20x Ph1 A-Plan objective.
A fully equipped Axioscop histology upright microscope (Zeiss) was used to image the histology staining. This system operates with an Axiocam HRc color digital camera (Zeiss) and Axiovision software for image acquisition. Images were acquired using a 5x, 10x or 20x objective in combination with the high resolution mode of 3900×3090 pixel of the camera.
The 3D collagen migration assay was imaged on an Eclipse Ti microscope (Nikon) equipped with perfect focus, motorized stage, a CoolSNAP HQ2 monochrome CCD camera (Photometrics) and a heating control. Imaging was performed with a 20x Plan Fluor objective. Since the microscope was not equipped with CO2 control the media was supplemented during imaging with 10 mM HEPES. MetaMorph software was used to image the cells every 10 min.
Assessment of membrane localization of the GFPKrasV12 construct, as well as high resolution imaging of the cellular morphology of highly GFPKrasV12-expressing cells were performed on a custom-built axially swept light sheet fluorescence microscope (Dean et al., 2015). GFPKrasV12-expressing cells were seeded on gelatin-coated glass coverslips and fixed. For morphology assessment, cells were additionally stained for Actin with 1:200 diluted PhalloidinAlexa594 (Invitrogen). 3D images were acquired with an isotropic pixel size of 160 × 160 × 160 nm. 3D volume renderings were performed using Amira 6.0.0 (FEI) with image brightness and contrast enhanced. For Movie3, the 3D image was rotated using Matlab (Mathworks) to align the direction perpendicular to the coverslip with the z-direction of the image, and for Movie6 the two channels were made equally semi-transparent.
Migration analysis
Migration velocity analysis was performed as described earlier (Schafer et al., 2012). Randomly selected cells that show motility were manually tracked. Cells within spheres were not tracked due to the limitations of accuracy of this method. Only cells that were tracked over a minimum of 100 min were used for velocity analysis. Imaging frequency for all experiments was 10 min. For the low and high KrasV12 clone comparison, low KrasV12 clones were imaged for 6 h. Since high KrasV12 clones moved slower and to still get a correct measurement, clones were imaged over a period of 14 h. In total 2 × 50 cells each low KrasV12 clone and 39 or 50 cells each high KrasV12 clone were used for analysis. To compare the migration velocities in low KrasV12 clone expressing GFP or GFPKrasV12, cells were imaged for 6 h. For each condition 59 cells were analyzed. To study the effect of ERK inhibition on high KrasV12 cells, migration was analyzed over a period of 4 h for control and for treated cells. 50 control cell and 40 treated cells were analyzed. To investigate the effect of Myosin inhibition in high KrasV12 cells, migration of 74 control and 80 treated cells were analyzed over the period of 6 h. The same time course was used to study the migration changes of low KrasV12 cells seeded on plastic dishes or on 15 kPa or 1.5 kPa soft ESS. Here, 60 cells on plastic, 57 cells on 15 kPa ESS and 60 cells on 1.5 kPa ESS were analyzed.
To quantify the migration behavior, tracks from the analysis described above were used and individual cell trajectories were linear-smoothened over 3 time-points (total 30 min). This was done to account for eventual inaccuracies during tracking. If a track showed one or more zero displacements, the cell was categorized as interrupted moving. Cells without stopping (all displacement values >0) were categorized as persistent. To make the data sets from low and high KrasV12 clones comparable, only the first 6 h of high KrasV12 clones were used for this analysis. Numbers of cells analyzed are indicated in each figure legend.
Protein lysis and western blotting
Cells were washed once with ice-cold PBS on the plate or in terms of spheres, spun down, re-suspended in PBS and again spun down for further lysis. Cells were lysed in ice-cold lysis buffer (50 mM Tris, 2% SDS and 10% Glycerol) supplemented with Complete Protease and PhosSTOP Phosphatase inhibitor cocktail (Roche). Protein concentrations were measured using the BCA protein assay kit (Pierce). For western blot analysis, 4–30 ug of protein were diluted in Laemmli-buffer (Bio-Rad) and loaded per well. Proteins were separated on a 4–20% gel (Bio-Rad) in running buffer (Bio-Rad) and transferred on PVDF or Nitrocellulose (Thermo Scientific) membrane using wet transfer box (Bio-Rad). Membranes were blocked in 5% non-fat dry milk powder (MP) in PBS containing 0.1% Tween (PBST). Primary antibody incubation was done overnight at 4°C. The following antibodies and dilutions in 5% MP/PBST were used: 1:20,000 or 1:5,000 anti-beta-Actin (Sigma, AC-15), 1:100 anti-Kras (Santa Cruz, SC-30), 1:400 anti-p53 (Santa Cruz, DO-1), 1:500 anti-p44/42 MAPK (T202/Y204, pERK, Cell Signaling, E10), 1:1,000 anti-FAK (Cell Signaling, D2R2E), 1:500 anti-RasV12 (Cell Signaling, D2H12). The following antibodies were diluted in 5% BSA/PBST: 1:1,000 anti-cMyc (Cell Signaling, D84C12), 1:1,000 anti-ERK 1 (Santa Cruz, C-16), 1:500 anti-pFAK (Y397, Cell Signaling, D20B1), and 1:200 anti-Myosin light chain 2 (Cell Signaling, D18E2) and 1:100 anti-P-Myosin light chain 2 (T18/S19, Cell Signaling, 3674S) were diluted in 5% BSA/TBST (Tris-buffered solution containing 0.1% Tween). Before incubation, these membranes were washed 2x for 5 min with TBST. After incubation, membranes were washed 3x for 10 min in 5% MP/PBST and then incubated for 1 h with 1:2,000 HRP-tagged secondary antibodies against mouse or rabbit (Jackson ImmunoResearch) accordingly diluted in 5% MP/PBST. Membranes were developed using ECL reagent and imaged with G-Box (SynGene). To blot for Actin as standard (if not run separately), membranes were stripped with stripping buffer (0.08 M Tris/HCl, 2% SDS, 0.7 % β-Mercaptoethanol) at 50°C for 30 min and then re-blotted. Ratio analysis was performed using the software Gene Tools (SynGene) as well as the gel analysis tool from ImageJ (Wayne Rasband, NIH). Only samples run on the same membrane were compared.
Soft-agar assay and colony analysis
HBECp53-cMyc-Kras were grown in 21% or in 2% O2 for 5 weeks before analysis by the soft-agar assay. H2887 was cultured in 21% or in 1% O2 for 8 weeks before putting into the soft-agar assay. In general, one bottom layer of 0.75% low-melting agar (Becton, Dickinson and Company) diluted in supplemented KSFM or RPMI 1640 was prepared in a 35-mm dish one day before cell seeding. To seed cells into the second layer, media/agar dilution was cooled down and kept at 42°C. 2,000 cells per dish were seeded. After the agar solidified, medium was added on top. Dependent on the pre-culture of the cells, the dishes were cultured in 21%, 2%, or 1% O2 for 1 month. During this time the media was exchanged regularly. After 1 month, the colonies were fixed with 4% PFA (Electron Microscopy Sciences) in Dulbecco’s PBS containing Ca2+ and Mg2+ (PBS, Sigma) for 1 h at 37°C. Afterwards, PFA solution was exchanged to PBS containing 30 mM Glycine (Sigma). Before imaging, colonies were stained with 1:500 Hoechst (Sigma) diluted in PBS for 1 h at 37°C followed by washing twice with PBS for 30 min. In a separate setup, big colonies from the HBECp53-cMyc-Kras soft-agar assay were isolated and put back in culture for further experiments.
For colony analysis, the experiments for each condition were performed in three replicates. For each replicate 10 positions of the dish were imaged as z-stacks through the soft-agar layer. The field of view per each z-stack was 4160×3510 μm and the z-step was 50 μm. Custom Matlab software was designed to measure the number and size of colonies imaged by fluorescence microscopy. The program first discards non-circular, very small, and very large objects in each image. To find these objects the maximum intensity projection (MIP) of each z-stack over the axial (z) direction was used. Since the colonies are sparsely distributed it was assumed that they did not overlap laterally in the MIPs. Next, the MIPs were bandpassed with a lower frequency of ~5 μm (3 pixels) and a higher frequency of ~31 μm (19 pixels) (Crocker and Grier, 1996), and then a Rosin threshold was applied to the image. Since the colony shape is only apparent by including pixels that are below the Rosin threshold, the bandpassed images were thresholded at 10% of the Rosin threshold. Non-circular objects such as dust were excluded by discarding objects in the Rosin thresholded image that had circularity below 0.7 in the image thresholded at the lower intensity level. Circularity was defined as the area divided by the perimeter squared and normalized such that a circle would have a circularity of 1. In the Rosin thresholded image objects were discarded whose areas were too small (~21 μm2) or too large (~4200 μm2). Next, the positions of the colonies were identified. To find the lateral (x and y) positions of the colonies, the intensity peaks in the bandpassed MIPs were located. For each object in the Rosin thresholded image, its position was defined as the location of the intensity peak within the object with the highest associated intensity value in the bandpassed MIPs. To find the axial (z) positions of the colonies, a box around each x-y position was constructed with a width of 19 pixels in the lateral dimensions and encompassing the entire image in the axial dimension. The z-position of the associated colony was defined to be the z-position of the voxel in the box with the greatest intensity. When finding the lateral colony position, the image was first bandpassed. Finally, the diameter of the colonies was measured. First, the effective background was subtracted from the raw images. This was done by averaging each raw image across the z-dimension, blurring the averaged image with a Gaussian filter with a standard deviation of ~31 μm (19 pixels), and then subtracting the blurred image from every z-plane of the raw data. To reduce noise this background-subtracted image was blurred in the z-plane of the colony with a Gaussian filter of width ~1.5 μm (1 pixel). The blurred image was thresholded with a global threshold set by the user. The same threshold value was used for all colony radii measurements. As before, objects with circularity below 0.7 were removed as well as very small (~21 μm2) and very large (4200 μm2) objects. The diameter of the colonies was estimated as the square root of the area in the focal plane of the colony divided by π/2.
Immunofluorescence
For polarity assessment, high and low KrasV12 clones were seeded onto gelatin-coated glass cover slips. For Kras staining in sphere attachment assay, spheres were transferred on collagen I pre-coated plates (Corning) to induce attachment for 24 h. All samples were fixed at the end of the experiment with 4% PFA diluted in Dulbecco’s PBS containing Ca2+ and Mg2+ (PBS) for 20 min at 37°C. Afterwards, samples were washed or stored until staining in 30 mM Glycine/PBS.
For staining of low and high KrasV12 clones for Myosin II, phosphorylated Myosin and Actin, cells were blocked for 1 h at room temperature with 10% Goat serum in PBS containing 0.3% Triton. Primary antibody against Myosin IIa (BioLegend, #PRB-440P) or against phosphorylated Myosin (P-Myosin Light Chain 2, S19, Cell Signaling, 3671) was diluted 1:400 or 1:50 respectively in 1% BSA/PBS containing 0.3% Triton and incubated over night at 4°C in a humidified chamber. Afterwards, the samples were washed 3x for 5 min with PBS plus 0.2% Tween-20. Secondary antibody against mouse conjugated with Alexa488 (Invitrogen) was diluted 1:500 and mixed with 1:200 Phalloidin Alexa594 (Invitrogen) and 1:1000 Hoechst in 1% BSA/PBS containing 0.3% Triton and incubated for 1 h at 37°C. Finally, the samples were washed 3x for 5 min in PBS/0.2% Tween-20 and once in MilliQ before mounting with Fluoromount.
For the sphere attachment assay, cells were permeablized for 10 min in 0.2% Triton/PBS, then washed once in 10% Goat serum in PBS containing 0.2% Tween-20 and then blocked for 2 h at room temperature with 10% Goat serum/PBS/0.2% Tween-20. Primary Kras antibody (Santa Cruz, SC-30) was diluted 1:50 in 10% Goat serum/PBS/0.2% Tween-20 and incubated over night at 4°C in a humidified chamber. Afterwards, the samples were washed 3x for 10 min with 5% Goat serum/PBS plus 0.2% Tween-20. Secondary antibody against mouse conjugated with Alexa594 was diluted 1:200 and mixed with 1:1000 Hoechst and in 10% Goat serum/PBS/0.2% Tween-20 and incubated for 1 h at 37°C. Finally, the samples were washed 3x for 10 min in PBS/0.2% Tween-20 and once in PBS before imaging.
Flow Cytometry
H2887 cells were cultured in 2% O2 over 4 weeks and fixed with 2% PFA in PBS for 20 min at 37°C. Afterwards cells were washed with 30 mM Glycine/PBS. Cells were permeablized for 2 min in 0.03% Triton/PBS, then washed once in PBS and blocked for 2 h at room temperature with 10% Goat serum in PBS. Primary Kras antibody (Abcam, ab55391) was diluted 1:50 in 10% Goat serum/PBS and incubated over night at 4°C in a humidified chamber. Afterwards, the samples were washed 3x for 10 min with 5% Goat serum/PBS plus 0.2% Tween-20. Secondary antibody against mouse conjugated with Alexa488 was diluted 1:500 in 10% Goat serum/PBS and incubated for 1 h at room temperature. Finally, the samples were washed 3x for 10 min in PBS/0.2% Tween-20 and once in PBS. Detached spheres were always collected by centrifugation and added back into the staining process. Finally, all cells were detached by Versene (Gibco) treatment and gentle scraping, collected, washed once and re-suspended for analysis in PBS. Kras intensities were analyzed using the BD FACS Aria II SORP (BD Biosciences) with a 488 nm excitation laser and a 530/30 band pass filter and gates were selected for viable cell population. Graphical analysis was performed using FlowJo v.10software (FLOWJO, LLC.).
Immunohistochemistry
Slides with paraffin-embedded human lung tumor tissue sections tested positive for KrasV12 mutation were obtained from the Cooperative Human Tissue Network (CHTN) following UT Southwestern IRB approval (IRB#: STU 102014-009). A 1:200 dilution of anti-Kras antibody (Abcam, ab55391) and a 1:80 dilution of Hif1α antibody (Novus, NB100-105) were used to stain for KrasV12 and Hif1α, respectively. 1:50 dilution of pERK (T202/Y204, pERK, Cell Signaling, E10), 1:50 dilution of pFAK (Y397, Cell Signaling, D20B1) and 1:100 dilution of pMLC (T18/S19, Cell Signaling, 3674S) were used. To compare KrasV12, Hif1α, pERK, pFAK and pMLC overlay, sequential slides were stained for Kras following Hif1α, pERK, pFAK and pMLC in the next consecutive sections. The Vectastain protocol provided for the Vectastain Elite PK-6102 kit (Vector Laboratories) was used for all immunohistochemistry experiments. Briefly, slides were heated at 57°C for 15 min and de-paraffinized by washing three times in Xylene for 5 min. Slides were then incubated in 100% Ethanol for 5 min followed sequentially by 2 min washes in 90%, 80%, 70%, and 50% Ethanol. Subsequently, slides were placed in water for 5 min to complete rehydration. Slides were then placed in sodium citrate (0.01 M sodium citrate dihydrate, 0.05% Tween, pH: 6.0) and boiled for 3 min for antigen presentation. Afterwards, slides were washed in water and equilibrated in TBST (0.02 M Tris, 0.1% Tween, 0.15 M NaCl, pH: 7.6). Endogenous peroxidase was blocked by incubating the slides in 0.3% H2O2 for 30 min. Slides were washed twice for 5 min in the TBST. Endogenous Biotin and Avidin were blocked using a Biotin/Avidin blocking kit (SP-2001, Vector Laboratories). Tissue sections were then blocked with horse serum for 1 h. Sections were treated overnight at 4°C with primary antibody prepared in blocking solution at dilutions described above. A high salt wash was performed twice for 5 min in TBST containing 0.3 M NaCl. Slides were treated with anti-mouse secondary antibody (provided by Vectastain Elite PK-6102 kit) diluted 1:200 in blocking solution for 30 min at room temperature. Slides were washed twice with TBST and treated with the Vectastain reagent for 30 min. Following 2 × 5 min washes in TBST, slides were developed by adding peroxidase substrate (ImmPACT DAB Peroxidase Substrate Kit, SK-4105, Vector Laboratories) and were observed immediately under a light microscope. The reaction was stopped by washing slides in water, and the time for development was kept consistent for all slides. Hematoxylin staining was performed once the slides were dry by incubating slides in Hematoxylin for 15 sec, followed by 2x washes with TBST and a final wash in water. Following drying, slides were covered with a cover-slip for imaging.
Nuclei aspect ratio measurements
Images of areas with low and high KrasV12 staining with lung tumor section from 5 patients were acquired and nuclei shape was assessed using ImageJ. Areas were assigned visually by intensity of brown Kras staining. No brown staining was defined as low KrasV12 and clear, strong brown signal was defined as high KrasV12 areas. The nuclei stained with Hematoxylin were outlined manually and the aspect ratios of the nuclei of all cells within the defined areas were measured in ImageJ. The following numbers of nuclei were analyzed for each patient: Patient1: low KrasV12: 587, high KrasV12: 172; Patient2: low KrasV12: 420, high KrasV12: 189; Patient3: low KrasV12: 222, high KrasV12: 131; Patient4: low KrasV12: 274, high KrasV12: 110; Patient5: low KrasV12: 283, high KrasV12: 87.
RNA isolation and KRAS droplet digital reverse transcription (RT) PCR
To analyze the levels of total (wild-type and mutated) KRAS mRNA changes upon long-term hypoxia culture in HBECp53-cMyc-Kras and H2887, we utilized droplet digital RT PCR (ddPCR). Cells were grown for 6 weeks in 21 or 1% O2. Afterwards, RNA was isolated from 5 independent repetition samples for each condition using the High Pure RNA Isolation Kit (Roche). RNA concentration was measured using the Synergy system (BioTek). Up-regulation on the protein level of KrasV12 was confirmed by western blot before ddPCR analysis. RNA was reverse transcribed (100 ng input) into first strand cDNA with iscript (Bio-Rad) in the presence of an exogenous spike-in control RNA for RT efficiency correction (bacteriophage MS2 RNA; Roche). Following cDNA synthesis, cDNAs were diluted 1:4 and 2 μl of diluted cDNA was mixed with a reaction containing primers and 5′ hydrolysis probes for both KRAS (F-5′-tggacgaatatgatccaacaat, R-5′-tccctcattgcactgtactcc; Universal Probe library #62-6FAM, Roche) and MS2 viral genes for assembly protein 1 (F-5′-GTCGCGGTAATTGGCGC, R-5′-GGCCACGTGTTTTGATCGA, probe-5′-HEX-AGGCGCTCCGCTACCTTGCCCT-bhq1) and the lysis protein (F-5′-CCTCAGCAATCGCAGCAAA, R-5′-GGAAGATCAATACATAAAGAGTTGAACTTC, probe-5′-HEX-CAAACATGAGGATTACCCATGTCGAAGACA-bhq1). Following droplet generation, PCR was performed with the following thermoprofile (95°C for 10 min for polymerase activation, 40 cycles of 94°C for 30 sec, 60°C for 1 min, 98°C for 1 min for polymerase inactivation and 20°C hold). Droplets were then read in a flow-cytometer like fashion and scored for absence or presence of fluorescence. Following correction of molecule counts for multiple templates per droplet (Poisson distribution correction) molecule per microliter counts are obtained for KRAS transcripts and exogenous MS2 assembly and lysis transcripts. To correct for RT efficiency differences between samples molecule counts were normalized to the average of both MS2 transcript levels. Following normalization to MS2, total molecule numbers were calculated by multiplying the molecules per microliter data by 20 (20 μl PCR volume). These data were expressed as molecules of KRAS RNA per 10 ng of input RNA.
Statistical analysis
Data representation was done using Excel (Microsoft). The Kolmogorov-Smirnov-test was used to test data sets for normality. This was done using the Prism6 software (GraphPad). If failed an unpaired, two-tailed Mann-Whitney test was applied as indicated in figure legends; otherwise, a two-sided, unpaired Student’s t-test was applied to determine the significance of differences between two data sets. For the comparison of multiple data sets a one-way, multi-comparison ANOVA-test was used. A P-value of <0.05 was assigned as significantly different and P-value of <0.05 was indicated as *, of <0.01 as ** and of <0.001 as *** in the figures.
Results
Long-term hypoxia promotes sphere-like morphology and increases KrasV12 protein levels in vitro and in vivo
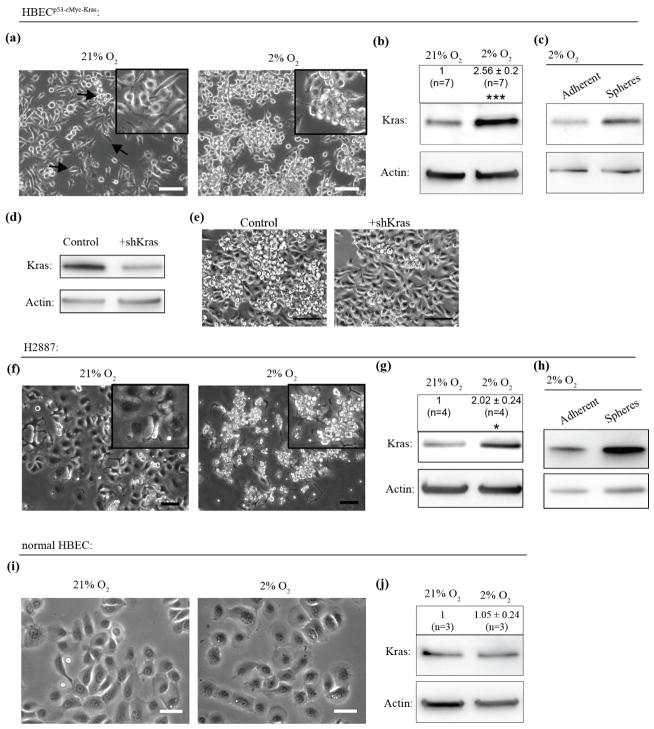
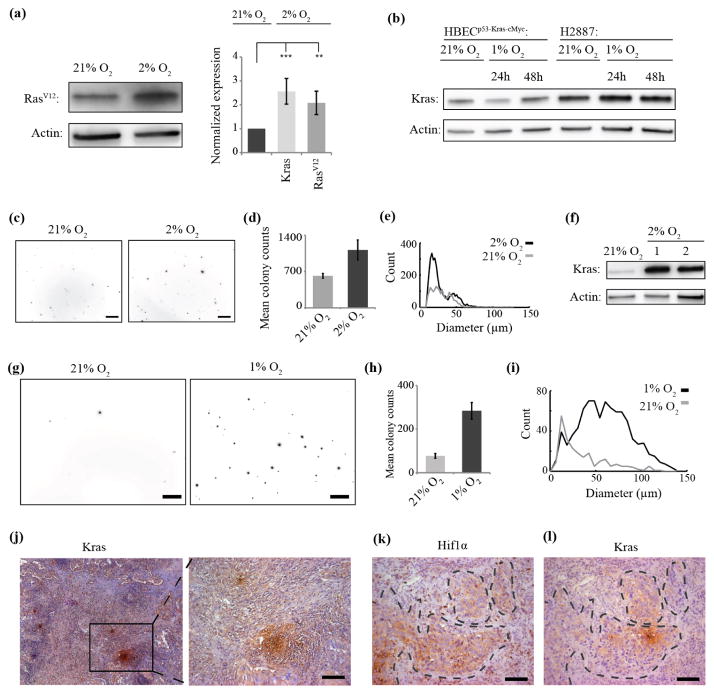
We first examined the effect of long-term hypoxia in an isogenic cell model of metastatic lung cancer, which had been derived from normal, immortalized (with hTERT and cdk4) human bronchial epithelial cells (HBEC) and transformed by knock-down of p53, and ectopic expression of cMyc and KrasV12 (HBECp53-cMyc-Kras). Under atmospheric (~21% O2) conditions these cells are morphologically heterogeneous (Sato et al., 2013) (figure 1(a), left). Upon long-term, hypoxic cultivation (>4weeks in 2% O2), we found that HBECp53-cMyc-Kras increasingly homogenized and assembled in sphere-like clusters (figure 1(a), right). This change was accompanied by an increase in KrasV12 protein expression levels (figure 1(b)). No cell death was observed due to long-term low Oxygen culture conditions. Careful separation of sphere-like clusters from adherent, spread cells within the same culture revealed that spheres exhibited higher KrasV12 expression (figure 1(c)). In addition, knock-down of Kras (wild-type and mutated) in HBECp53-cMyc-Kras abrogated the sphere-like phenotype (figure 1(d), (e)). The same morphological changes (figure 1(f)), as well as an increase in KrasV12 expression (figure 1(g)), specifically in the spheres (figure 1(h)), was observed under long-term hypoxic culture in the patient-derived non-small cell lung carcinoma cell line H2887 harboring the KrasV12 mutation. Untransformed HBECs bearing wild-type Kras did not show changes in either morphology (figure 1(i)) or Kras expression (figure 1(j)) under hypoxic conditions, assigning this effect specifically to mutated Kras-transformed cancer cells. Upregulation of the mutated KrasV12 in HBECp53-cMyc-Kras upon hypoxia was confirmed using a mutation-specific antibody (figure 2(a)). Direct comparison of total Kras (wild type and mutated) and only mutated KrasV12 protein levels determined KrasV12 being upregulated to hypoxia. Together, these data suggest a correlation between hypoxia, KrasV12 expression, and cell morphology. To further characterize the hypoxia-driven increase in KrasV12 expression, we first confirmed that short-term (24–48 h) exposure to low Oxygen did not lead to increased KrasV12 protein levels in HBECp53-cMyc-Kras and H2887 cells (figure 2(b)). Direct comparison of the effect of hypoxia on KrasV12 protein (supplementary figure 1(a)) and on mRNA levels in HBECp53-cMyc-Kras as well as in H2887 harboring intrinsic KrasV12 mutation (supplementary figure 1(b)) revealed that the up-regulation in both cell lines is controlled at the protein level suggesting interference between hypoxia and translation of KrasV12 protein recycling- and/or degradation. Importantly, neither cMyc nor p53 levels changed in HBECp53-cMyc-Kras upon long-term 2% O2 culture in both cell lines, excluding a role of cMyc nor p53 protein levels in the observed morphology changes (supplementary figure 1(c), (d)). Since expression of the oncogenic form of Kras is related to increased tumorigenic potential (Pylayeva-Gupta et al., 2011), we also performed soft-agar colony formation assays with HBECp53-cMyc-Kras and H2887 cells (figure 2(c), (g)) to test whether hypoxia-induced KrasV12 up-regulation promotes tumorigenicity. Indeed, both cell lines showed an increase in the number (figure 2(d), (h)) and size (figure 2(e), (i)) of the colonies under low Oxygen conditions. The increase went along with increase in KrasV12 expression levels in the colonies (figure 2(f)), indicating a central role of KrasV12 protein concentration for in vitro tumorigenicity.
Figure 1. Long-term hypoxia increases oncogenic KrasV12 expression levels and sphere-like morphology.
(a), Human bronchial epithelial cells transformed with p53 knock-down, KrasV12 and cMyc over-expression (HBECp53-cMyc-Kras) change morphological heterogeneity upon long-term (> 4 weeks) cultivation in 2% O2 compared to 21% O2. Different cellular morphologies are indicated by black arrows. Scale bar: 100 μm. (b), KrasV12 expression levels increase upon long-term cultivation in 2% O2. (***: P < 0.001 by Mann-Whitney test, ±: standard error). n indicates number of data points. (c), Spheres are higher in KrasV12 protein levels. (d), KrasV12 levels decrease after knock-down using shRNA against total (wild-type and mutated) Kras in HBECp53-cMyc-Kras cultured in 2% O2. (e), This treatment leads to decrease of sphere-like morphology. Scale bar: 100 μm. (f), Lung cancer cell line H2887 bearing KrasV12 mutation shows morphological heterogeneity grown in 21% O2 with adherent and sphere-like cells. Upon long-term cultivation in 2% O2 cellular sphere-like morphology increases. Scale bar: 100 μm. (g), KrasV12 levels significantly increase in long-term hypoxia. (*: P < 0.05 by Mann-Whitney test, ±: standard error). n indicates number of data points. (h), Higher KrasV12 protein levels in spheres. (i), Normal, untransformed HBECs show homogeneous morphology which does not change upon long-term cultivation in 2% O2. Scale bar: 50 μm. (j), Long-term hypoxia does not affect Kras protein levels. (non-significant by Mann-Whitney test, ±: standard error). n indicates number of data points.
Figure 2. Upregulation of KrasV12 in long-term hypoxia increases tumorigenicity in vitro and is correlated with hypoxia areas in vivo.
(a), RasV12-specific antibody was used to confirm KrasV12 upregulation in HBECp53-cMyc-Kras upon 2% O2. Quantification comparing total Kras and mutated KrasV12 shows similar increase in 2% O2 indicating major role of hypoxia-induced KrasV12 upregulation. Error bars show standard error. (**: P < 0.01, ***: P < 0.001 by ANOVA-test). (b), Short-term (24–48 h) exposure to hypoxia does not significantly increase KrasV12 expression in HBECp53-cMyc-Kras and H2887. (c), Maximum intensity projections of z-stacks of soft-agar colonies formed in 21% or 2% O2 show increased tumorigenic potential upon hypoxia in HBECp53-cMyc-Kras. Scale bar: 500 μm. (d), Number of soft-agar colonies of HBECp53-cMyc-Kras increases in 2% O2. (n=3, error bars show standard deviation). (e), The proportion of larger colonies of HBECp53-cMyc-Kras increases in 2% O2. (f), Analysis of KrasV12 levels shows high levels for two soft-agar colonies isolated from the 2% assay compared to HBECp53-cMyc-Kras grown in 21% O2. (g), Maximum intensity projections of z-stacks of soft-agar colonies formed in 21% or 1% O2 show increased tumorigenic potential upon hypoxia in H2887 cells. Scale bar: 500 μm. (h), The number of soft-agar colonies of H2887 cells increases in 1% O2. (n=3, error bars show standard deviation). (i), The proportion of larger colonies of H2887 cells increases in 1% O2. (j), KrasV12 expression is heterogeneous in vivo as observed by staining for Kras in lung tumor sections positive for KrasV12 mutation. Scale bar: 100 μm. (k), (l), Localization of KrasV12 hotspots overlays with hypoxic areas in the tumor as identified by Hif1α (k) and Kras (l) staining in consecutive lung tumor slides. Hif1α positive areas were outlined (k) and overlay with Kras in (l). Scale bars: 100 μm.
Our in vitro data provide strong evidence that KrasV12 expression levels are sensitive to long-term hypoxia and that such increase in KrasV12 can trigger a profound switch in cell morphogenesis. We next tested if this may also apply in vivo. For this purpose, lung tumor sections from 5 patients tested positive for KrasV12 were stained for Kras. All sections displayed a heterogeneous KrasV12 expression pattern with hotspots of high KrasV12 expression levels throughout the tumor (figure 2(j)). To test whether these hotspots correlate with hypoxic areas in the tumor mass we stained sequential tumor sections for Kras or for Hif1α, which is a marker for hypoxic areas (figure 2(k)). Indeed, we found that neighbor tumor sections showed same areas positive for KrasV12 and Hif1α expression in all 5 patients (figure 2(k), (j) and supplementary figure 1(e)). We further wanted to test if this localized coupling of KrasV12 expression and hypoxia would be associated with morphological changes akin to those observed with high KrasV12 expression in vitro. For these analyses, areas of low and high KrasV12 staining within the same tumor were identified (supplementary figure 2(a)–(c)) and the aspect ratio (ratio between the long and short axes of an ellipse approximating the nuclear outline) of the nuclei (supplementary figure 2(d)) was used as a proxy for cellular morphology (Irshad et al., 2014, Sanchez-Laorden et al., 2014). Regions with high or low KrasV12 staining displayed a significant difference in nuclear shape: the former contain round nuclei (aspect ratio ~1), the latter contain elongated nuclei (aspect ratio ≫1) (supplementary figure 2(e), (f)). Together, these histological analyses support the correlation between hypoxia, KrasV12 protein levels and cell morphogenesis. All the following experiments were conducted under hypoxic conditions.
Differential KrasV12 protein levels are associated with clonal morphology and migration behavior
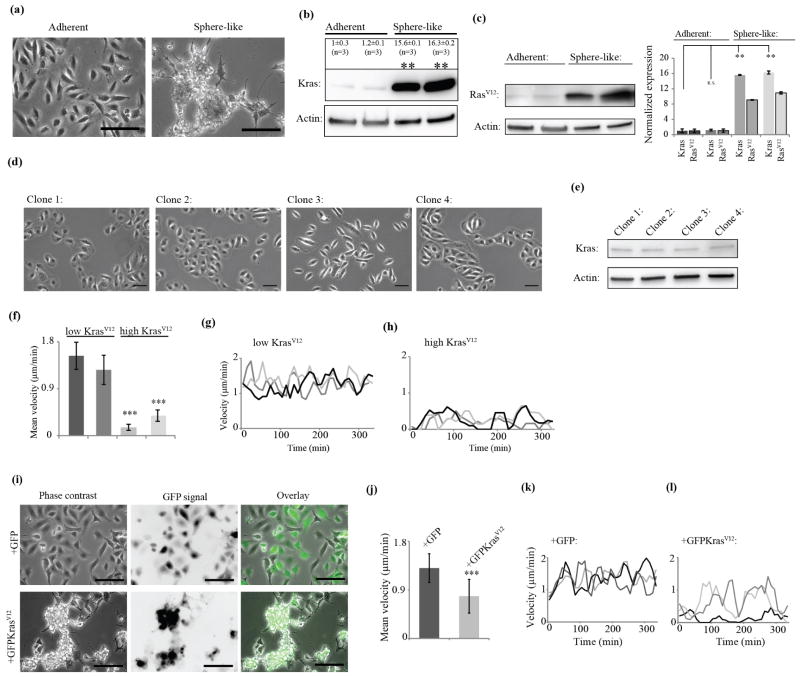
First, we investigated the mechanism by which differential expression levels of constitutively active KrasV12 signal translate into different cell morphological programs. We isolated single clones from HBECp53-cMyc-Kras in 2% O2 culture conditions and obtained stable clones with an adherent, spread morphology and similarly stable clones with a predominant sphere-like, non-adherent morphology (figure 3(a)). These morphologically selected clones differed vastly in Kras expression (figure 3(b)). Knock-down of Kras in HBECp53-cMyc-Kras led to a reduction in the sphere-like phenotype (figure 1(d), (e)). Comparison of Western blots using generic versus mutation-specific Kras recognizing antibodies confirmed the involvement of the mutant form in the sphere-like clones (figure 3(c)). The mutant-specific antibody was tested in different lung cancer cell lines bearing different Kras mutations to determine background and specificity of the antibody (supplementary figure 3(a)). No signal was detected in cell lines negative for the KrasV12 mutation confirming the presence of KrasV12 protein in the low KrasV12 clones. Moreover, no differences in cMyc expression were observed between the clones, supporting a specific association between KrasV12 expression and the observed morphological changes (supplementary figure 3(b)). Clonal separation of untransformed HBECs harboring only wild-type Kras neither produced morphological heterogeneity (figure 3(d)) nor showed variation in Kras protein levels (figure 3(e)). This underlines the dependence of the morphological switches in HBECs on a transformation with oncogenic KrasV12.
Figure 3. Differential levels of oncogenic KrasV12 control cell morphology and migration.
(a), Clonal separation of HBECp53-cMyc-Kras reveals clones with adherent and sphere-like morphologies. Scale bar: 100 μm. (b), Morphologies of clones correlate with Kras protein levels. (**: P < 0.01 by ANOVA-test, ±: standard error). n indicates number of data points. (c), Expression of KrasV12 was confirmed using a mutation specific RasV12 antibody in adherent and sphere-like clones. Comparison of KrasV12 (using a RasV12-specific antibody) and general Kras levels (using an antibody recognizing wild type and mutated forms) indicates that the expression increase between adherent and sphere-like clones is predominantly related to expression increase of the mutant form. Error bars show standard error. (**: P < 0.01 by ANOVA-test, n.s.: not significant, n=3, for RasV12 of first low KrasV12 clone: n=2). (d), Clonal isolation of normal, immortalized HBECs (Human Bronchial Epithelial Cell) produced clones with homogeneous, adherent morphology. Scale bar: 50 μm. (e), Kras levels are stable between clones from normal HBECs. (f), Analysis of random migration of high KrasV12 clones (n=39 and 50) and low KrasV12 clones (both n=50). (***: P < 0.001 by Student’s t-test). Error bars show standard deviation. (g), Migration velocities of three representative cells from a low KrasV12 clone are displayed. (h), Migration velocities of three representative cells from a high KrasV12 clone are displayed. (i), Morphology of adherent, low KrasV12 clones isolated from HBECp53-cMyc-Kras switches to sphere-like upon exogenous over-expression of GFPKrasV12. The switch is not observed when infected with empty GFP vector as control. Scale bar: 100 μm. (j), Comparison of velocities of low KrasV12 clones infected with either empty GFP vector (n=59) or GFPKrasV12 (n=59). (***: P < 0.001 by Mann-Whitney test). Error bars show standard deviation. (k), Migration velocities of three representative cells from a low KrasV12 clone infected with GFP control vector. (l), Migration velocities of three representative cells from a low KrasV12 clone infected with GFPKrasV12 are displayed. (a)-(l) All experiments were performed in 2% O2.
To analyze the migration behavior of HBECp53-cMyc-Kras clones, we imaged and tracked random, single cell migration over the time course of 6 h under 2% O2 conditions. Low KrasV12 clones migrated with a mean velocity between 1.3 and 1.6 μm/min (figure 3(f)). The movement was persistent (figure 3(g), only 1 out of 50 cells intermittently stopped migration, supplementary figure 3(c), Movie1). High KrasV12 clones grew mostly in sphere-like clusters with little observable movement. However, when cultured for longer they began to shed cells that subsequently adhered to the surface and initiated motility (Movie2). Even though these cells formed adhesions and started to migrate and thus likely differed from cells inside clusters in terms of KrasV12 levels, they showed markedly reduced motility compared to low KrasV12 clones, both in mean velocity and persistence (figure 3(f), (h); 38 out of 39 tracked cells showed an interrupted movement with at least one complete stop throughout the observation window; supplementary figure 3(c), Movie2). From this we conclude that low and high KrasV12 clones differ in their activation of the cell motility machinery and their ability to maintain stable polarity.
We next tested if motile low KrasV12 clones could be converted into sphere-forming, non-motile cells by ectopically inducing high KrasV12 levels. Low KrasV12 clones in 2% O2 culture conditions were stably infected with either a CMV-driven GFPKrasV12 to accomplish high KrasV12 levels or an empty control GFP vector. Membrane localization of the GFPKrasV12 construct was confirmed by 3D light sheet fluorescence microscopy (Movie3). Introduction of high levels of KrasV12 expression into low KrasV12 clones fully recapitulated the phenotype observed in high KrasV12 clones (figure 3, Movie4, 5). GFPKrasV12-expressing cells adopted a sphere-like morphology (figure 3(i), Movie6) and their mean migration velocity was significantly reduced (figure 3(j)) along with a decrease in migration persistence (figure 3(k), (l)) (increase of interrupted trajectories from 2% to 18%, 11 out of 60 cells, supplementary figure 3(d)). These data corroborate the roles of KrasV12 expression levels in cell morphology and motility control.
KrasV12 expression differentially activates a FAK/ERK/Myosin signaling
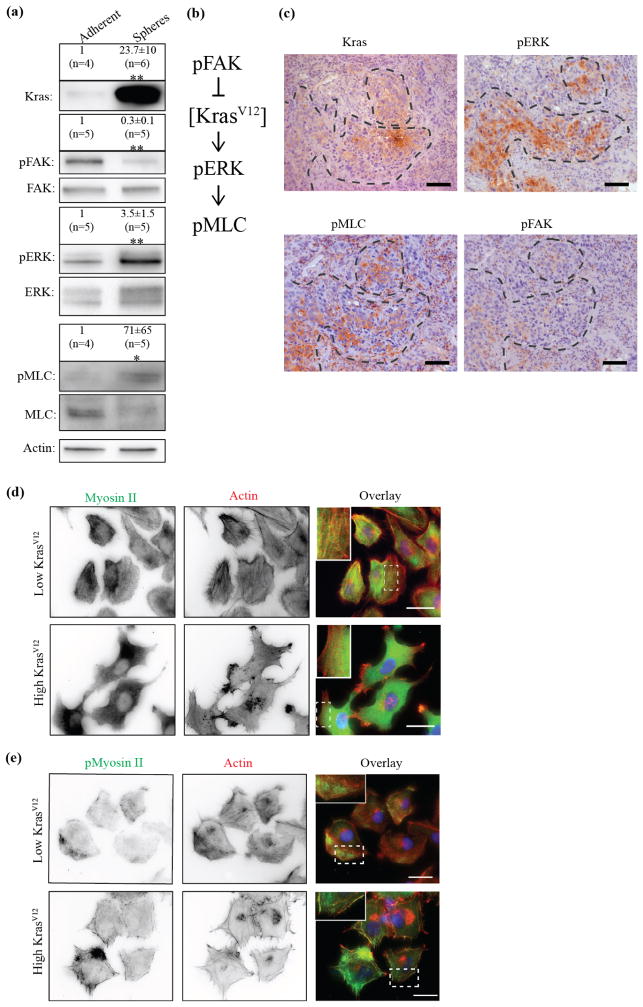
Next, we asked which signaling pathways could translate KrasV12 expression levels to differential morphology. Since the two phenotypes showed differences in adhesive properties we first tested the coupling of KrasV12 expression levels and cell adherence. We cultured HBECp53-cMyc-Kras either in adherent or in low attachment cell culture dishes in 2% O2 culture conditions. Low attachment plates initiated an exclusively sphere-like morphology of the cell line accompanied by increased KrasV12 levels (figure 4(a)). As expected (Mitra et al., 2005), FAK activity in high KrasV12-expressing spheres on low attachment dishes was much reduced, as seen in a decrease in FAK phosphorylation (figure 4(a)). Moreover, Kras is upstream of ERK activity (Rajalingam et al., 2007), which is a potential regulator of cell morphology through its capacity of activating Myosin II (Brahmbhatt and Klemke, 2003). High Myosin activity could promote a round, sphere-like phenotype (Lomakin et al., 2015). Therefore, we analyzed both ERK and Myosin II activation and indeed found both upregulated in high KrasV12 spheres (figure 4(a)). Based on these data we envisioned a pathway where adhesion signaling modulates the concentration of constitutively active KrasV12, which in turn controls the level of ERK and Myosin activity (figure 4(b)). Differential activation between adherent and sphere-like cells was confirmed in the patient-derived lung cancer cell line H2887 (supplementary figure 3(e)). Moreover, staining for pERK, pMLC and pFAK in human lung tumor sections support the notion of a differential activation of the proposed pathway in high versus low KrasV12–expressing tumor areas (figure 4(c)). pERK as well as pMLC were both activated heterogeneously throughout the tumor similarly to the observed KrasV12 distribution whereas pFAK staining was low. The staining was performed on consecutive sections. Therefore, a direct overlay of the signal was not expected.
Figure 4. Differential activation of FAK/ERK/Myosin II signaling pathway and altered cellular polarity and cytoskeleton distribution in high and low KrasV12 clones.
(a), Comparison of signaling profile of adherent and sphere-like HBECp53-cMyc-Kras population. In a western blot, high KrasV12 spheres show less FAK activation (pFAK/FAK) but increased ERK (pERK/ERK) and Myosin II (pMLC/MLC, MLC: Myosin Light Chain) activity. (*: P < 0.05, **: P < 0.01 by Mann-Whitney test, ±: standard error). n indicates number of data points. (b), Proposed signaling cascade consistent with data in (a). (c), Consecutive lung tumor sections tested positive for KrasV12 mutation were stained for pERK, pMLC and pFAK and compared to areas positive for KrasV12 expression (same picture also shown in figure 2(l)). Areas positive for KrasV12 signal show elevated staining of pERK and pMLC and low staining for pFAK. Conserved tumor structures throughout the slides are indicated with the dashed line. Scale bars: 100 μm. (d), Cells from low or high KrasV12 clones were fixed and stained for Myosin II (green in overlay) and Actin (red in overlay). Nuclei were visualized by Hoechst (blue in overlay). Low KrasV12 cells show typical polarization for effective migration with Actin-rich lamellipodium in the front and actomyosin-rich back. Such polarity is lost in high KrasV12 cells which show multiple lamellipodial initiation sites and an asymmetric Myosin II distribution. Zoomed inlets show punctuated Myosin localization along actin filaments. Scale bars: 20 μm. (e), Similar distribution is also observed by staining for phosphorylated Myosin (pMyosin II). Scale bars: 20 μm. (a), (d), (e) All experiments were performed in 2% O2.
KrasV12 levels associate with a switch in FAK/ERK/Myosin II activation
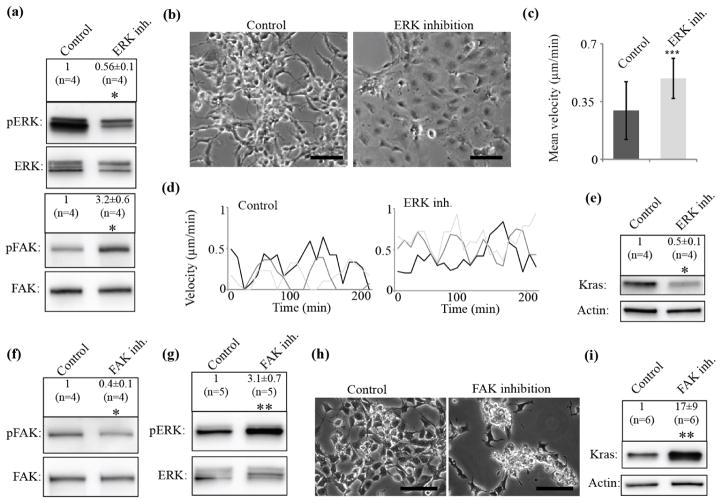
Our migration analysis showed big differences in the ability to maintain persistent movement in low versus to high KrasV12 clones (figure 3(g, h), supplementary figure 3(c)). The lack of polarity with one clearly defined lamellipodium could cause the reduced average velocity and persistence of single cell motility in these clones. Low KrasV12 clones with reduced ERK and Myosin II activity displayed robust separation of a well-defined actin-rich lamellipodia at the cell front and an actomyosin-rich rear, whereas high KrasV12 clones with high ERK and Myosin II activity lacked clear asymmetry in Myosin distribution and displayed multiple sites of cryptic lamellipodia (figure 4(d)). Similar distributions were also observed with staining for phosphorylated Myosin (figure 4(e)). Based on this data we expected that the morphological switches from high to low KrasV12 expression could be reproduced by direct inhibition of Myosin II or ERK. To test this, we first treated high KrasV12, sphere-like clones with the specific ERK inhibitor SCH772984 (Morris et al., 2013). This resulted in a decrease in ERK phosphorylation (figure 5(a)) which was comparable with the activation levels observed in the adherent, motile phenotype (compare figure 4(a)). In support of our hypothesis, this treatment led to a striking conversion of sphere-like into adherent cell morphology (figure 5(b)), accompanied by significantly faster (figure 5(c)) and more persistent movements (figure 5(d), 85% up from 8% of cells moved persistently after ERK inhibition, supplementary figure 3(f), Movie7, 8). At the same time activation of adhesion signaling as measured by FAK phosphorylation was observed (figure 5(a)), consistent with the notion that motile cells display increased mechanical engagement of integrin-mediated matrix adhesions. Most critically, we found that KrasV12 levels significantly decreased in these cells (figure 5(e)). Thus, inhibition of ERK reduced the expression level of the mutated KrasV12 protein presumably dependent upon adhesion engagement. To test directly the existence of a mechano-transductory feedback we disrupted adhesion signaling by selective inhibition of FAK using the small molecule compound FAK inhibitor 14 (Tocris). This treatment resulted in decreased FAK phosphorylation (figure 5(f)) comparable with the decrease observed in the sphere-like phenotype (compare figure 4(a)) and led to conversion of adherent clones into cells with a sphere-like morphology (figure 5(h)) and elevated KrasV12 expression (figure 5(i)). Along with this switch, ERK signaling was activated (figure 5(g)). Altogether, these data put KrasV12 expression levels into a double-negative feedback loop, in which ERK activation downstream of high concentrations of constitutively active KrasV12 promotes Myosin II activity that blocks cellular polarization and cell adhesion. Upon decrease of either KrasV12 concentration or ERK signaling, cells can spread and induce adhesion signals that reduce KrasV12 concentration further by a yet to be determined mechanism.
Figure 5. Reduction of ERK and FAK signaling converts clonal phenotypes and KrasV12 levels.
(a), High KrasV12 cells were treated with DMSO as control (Control) or with SCH772984 (ERK inh.), a specific ERK inhibitor, and protein extracts were analyzed for ERK activation by western blot and ratio analysis of phosphorylated ERK (pERK) and total ERK (ERK). Treatment with ERK inhibitor led to decrease in ERK activation. Such treatment also led to activation of FAK (focal adhesion kinase) signaling measured by western blot and ratio analysis of phosphorylated FAK (pFAK) to total FAK (FAK). (*: P < 0.05 by Mann-Whitney test, ±: standard error). n indicates number of data points. (b), Treatment of high KrasV12 sphere-like clones with the ERK inhibitor (ERK inh.) causes a switch to adherent morphology. Scale bar: 100 μm. (c), Comparison of migration velocities of control (n=50) and ERK inh. treated (n=40) cells. (***: P < 0.001 by Mann-Whitney test). Error bars show standard deviation. (d), High KrasV12 cells (control) switch migration behavior to more persistent movement upon ERK inhibition (ERK inh.). Shown are migration velocities of three representative cells from control or ERK-inhibited high KrasV12 cells. (e), KrasV12 levels decrease upon ERK inh. (*: P < 0.05 by Mann-Whitney test, ±: standard error). n indicates number of data points. (f), Low KrasV12 cells were treated with DMSO as control (Control) or with a specific FAK (focal adhesion kinase) inhibitor (FAK inh.), and protein extracts were analyzed for FAK signaling by western blot and ratio analysis of phosphorylated FAK (pFAK) and total FAK (FAK). Treatment with FAK inhibitor led to decrease in FAK activation. (*: P < 0.05 by Mann-Whitney test, ±: standard error). n indicates number of data points. (g), Such treatment also led to activation of ERK signaling measured by western blot and ratio analysis of phosphorylated ERK (pERK) to total ERK (ERK). (**: P < 0.01 by Mann-Whitney test, ±: standard error). n indicates number of data points. (h), FAK inhibition in low KrasV12 adherent clones leads to sphere formation. Scale bar: 100 μm. (i), Increase in KrasV12 expression levels upon FAK inhibition. (**: P < 0.01 by Mann-Whitney test, ±: standard error). n indicates number of data points. (a)-(i) All experiments were performed in 2% O2.
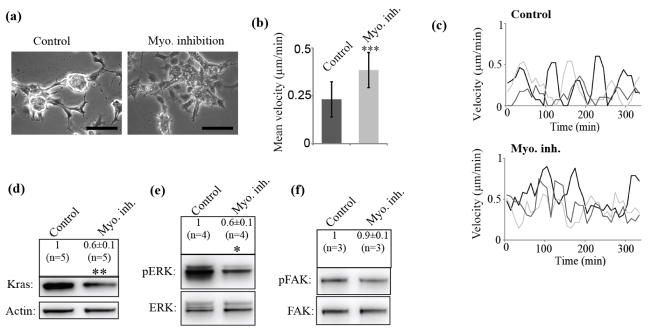
Next, we tested whether we could control KrasV12 levels and associated FAK/ERK switches by increasing and decreasing Myosin activity. We first reduced Myosin II activity in high KrasV12 clones by applying the Myosin II-specific ATPase inhibitor blebbistatin. This led to the formation of more adherent, spread cells (figure 6(a)) with significantly increased migration velocity (figure 6(b)) and persistence (figure 6(c), increase from 4% to 69% cells with persistent motion, supplementary figure 3(g), Movie9, 10). Importantly, decrease in Myosin II activity reduced the KrasV12 concentration levels (figure 6(d)) and ERK activation (figure 6(e)) whereas FAK phosphorylation was unaffected (figure 6(f)). Reduction of ERK was also in the same range as already detected before in the adherent phenotype (figure 4(a)). Complementary, activation of Myosin II by treatments of adherent, low KrasV12 clones using CalyculinA (CalA), switched the cells to a sphere-like phenotype (supplementary figure 3(h)) without significant reduction in FAK activity (supplementary figure 3(j)), but significantly increased KrasV12 levels (supplementary figure 3(i)) and ERK phosphorylation (supplementary figure 3(k)). The CalA experiments may be difficult to interpret by themselves since CalA is not a specific Myosin activator; nonetheless, the consistency of the results with the blebbistatin experiments is noteworthy. Together these data support the double-negative feedback model in which the two cell morphologies and KrasV12 levels could be interconverted by manipulation of ERK, Myosin II or FAK.
Figure 6. Myosin inhibition switches clonal phenotype to adherent and motile with decreased KrasV12 levels.
(a), Treatment of high KrasV12 sphere-like clones with Myosin inhibitor causes formation of more adherent phenotype. Scale bar: 100 μm. (b), Analysis of migration velocities of control (n=74) and Myosin inhibitor-treated (Myo. inh.) cells (n=80). (***: P < 0.001 by Mann-Whitney test). Error bars show standard deviation. (c), High KrasV12 cells switch migration behavior to more persistent movement upon Myosin II inhibition (Myo. inh.). Shown are migration velocities of three representative cells from control or blebbistatin-treated high KrasV12 cells. (d), This went along with a decrease in KrasV12 levels. (**: P < 0.01 by Mann-Whitney test, ±: standard error). n indicates number of data points. (e), Such treatment led to reduction in ERK activation measured by western blot and ratio analysis of phosphorylated ERK (pERK) to total ERK (ERK). (*: P < 0.05 by Mann-Whitney test, ±: standard error). n indicates number of data points. (f), No change of FAK signaling was observed by western blot and ratio analysis of phosphorylated FAK (pFAK) and total FAK (FAK). (±: standard error). n indicates number of data points. (a)-(f) All experiments were performed in 2% O2
KrasV12 levels respond to changes in the mechanical properties of the micro-environment
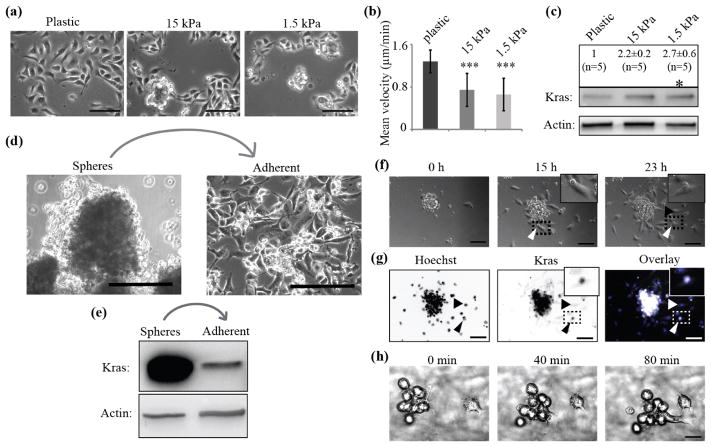
Another way to potentially interfere with adhesion engagement is by alteration of the mechanical micro-environment (Charras and Sahai, 2014). It is well established that adhesion engagement is more effective on stiff compared to soft substrates (Chan and Odde, 2008). Indeed, ECM remodeling within the tumor and its surroundings is a clinical hallmark of cancer progression (Hanahan and Weinberg, 2011); and ECM alignment and stiffness increase correlate with more efficient metastasis and tumor progression (Acerbi et al., 2015, Levental et al., 2009). To investigate the putative connection between mechano-transduction and KrasV12 expression, we first seeded low KrasV12 clones on elastically supported surfaces with decreasing stiffness (28, 15 or 1.5 kPa, representing a stiffness range of tissue and matrix in vivo (Discher et al., 2005)), where we observed less adhesion engagement than on infinitely stiff cell culture plastic (supplementary figure 4(b)). With decreasing substrate stiffness these low KrasV12 clones formed round, sphere-like clusters (figure 7(a), supplementary figure 4(a)). The remaining unclustered cell population appeared smaller and exhibited markedly lower mean migrating velocity (figure 7(b), Movie11–13). Population-wide analysis of the signaling state in this mixed culture showed that the lower mechanical engagement of adhesions led to a significant decrease in FAK phosphorylation (supplementary figure 4(b)), which was sufficient to increase KrasV12 expression levels on 15 and 1.5 kPa stiff surfaces and modest ERK activation (figure 7(c), supplementary figure 4(c)). On surfaces with a stiffness of 28 kPa no increase in KrasV12 was observed (supplementary figure 4 (d)). These findings were confirmed using the H2887 cell line where the switch in signaling and KrasV12 levels is even more pronounced (supplementary figure 4(e) – (g)). Cell morphology, FAK and ERK signaling as well as KrasV12 levels were all changed on softer substrates. We also performed the reverse experiment: High KrasV12 levels were initiated by culture of HBECp53-cMyc-Kras in low attachment dishes followed by transfer of sphere-like clusters onto plastic dishes. This induced cellular morphology changes from exclusively sphere-like to mostly adherent populations (figure 7(d)) accompanied by a strong decrease in KrasV12 levels (figure 7(e)). To more directly test the effect of different ECM densities on cell morphologies in low compared to high KrasV12 clones, we seeded such clones on micro-patterned chips that prevented cluster formation and were coated with different Collagen I concentrations (5–200 μg/ml). After 1 h of spreading (supplementary figure 4(h)), low KrasV12-expressing clones gave rise to a higher proportion of adherent cells compared to high KrasV12-expressing clones, which showed significantly more sphere-like cells for all Collagen I concentrations (supplementary figure 4(i)). Altogether, these manipulations support the notion of KrasV12 expression levels being controlled by an adhesion-dependent, mechano-sensitive switch.
Figure 7. Oncogenic KrasV12 expression is controlled by a mechano-sensitive switch determining cell morphology and migration.
(a), Adherent, low KrasV12 clones on surfaces with varying stiffness (plastic, 15 kPa and 1.5 kPa) form aggregates on soft (15 and 1.5 kPa) but not on stiff (plastic) substrates. Scale bar, 100 μm. (b), Migration velocities of adherent clones on 15 kPa (n=57) or 1.5 kPa (n=60) substrates compared to plastic (n=60). (***: P < 0.001 by Student’s t-test). Error bars show standard deviation. (c), KrasV12 levels increase on soft substrates. (*: P < 0.05 by ANOVA-test, ±: standard error). n indicates number of data points. (d), Transfer of high KrasV12 spheres onto attachment, plastic dishes initiates adherent phenotype and migration. Scale bar, 100 μm. (e), Culture on adhesive surfaces leads to reduction in KrasV12 expression. (f), Live cell imaging of dissemination from spheres over a time period of 23 h shows rapid initiation of adhesion and motility. Non-motile, round cells get transported out by adherent, motile cells which stay connected over the whole period of observation (arrow heads, zoomed inlet, Movie14). Scale bar, 50 μm. (g), After 23 h cells were fixed and stained for nuclei (Hoechst, blue) and Kras (white). KrasV12 levels in non-motile, cargo cells are high (arrow heads, zoomed inlet). Scale bar, 50 μm. (h), Live cell imaging in 3D collagen matrix shows conjoint migration of clusters of motile, spread and non-motile, spherical cells (Movie16). Scale bar, 50 μm. (a)–(g) All experiments were performed in 2% O2.
Finally, we wondered whether the separation of cells in two discrete morphological states associated with and low and high KrasV12 expression would also occur with a population of patient-derived H2887 cells (supplementary figure 4(j)). To test this, H2887 cells grown under 2% O2 conditions on adherent plastic dishes were fixed and stained for their intrinsic KrasV12 levels without interfering with their adhesion state. KrasV12 expression levels were then measured by flow cytometry. We found two separable populations with high and low KrasV12 levels (supplementary figure 4(j)). In addition, we consistently observed a small third population of cells with very high KrasV12 expression. In sum, these analyses showed that high and low KrasV12 expression is driven by the degree of mechano-transduction through cell-matrix adhesions.
Clusters of cells with high and low KrasV12 levels migrate together as a multi-functional unit
Live cell imaging of cell dissemination from high KrasV12 spheres showed a conversion to low KrasV12, adherent and motile cell populations within a 23 h time period that resulted in cells leaving the sphere (figure 7(f), (g), Movie14). Curiously, we frequently observed small cell collectives, where one motile low KrasV12 expressing cell drags along one or several round, high KrasV12-expressing cells (figure 7(f), (g), arrows, Movie14, 15). Such collectives of motile, spread and sphere-like cells were unveiled also by live cell imaging in a 3D Collagen environment (figure 7(h), Movie16). Interestingly, in this experiment we were able to observe the two cellular states, here purely judged on cell morphology and motility, in a 3D migration assay.
Discussion
Our data show that the concentration of a constitutively active signal, here of the oncogene KrasV12 can modulate divergent cell functions, allowing tumor cells to adopt plastic behavior (supplementary figure 5). We demonstrate that low levels of KrasV12 initiate an adherent and motile phenotype whereas high levels of KrasV12 protein induce a sphere-like, tumorigenic cell phenotype. Similar changes in morphogenesis were previously observed by introducing KrasV12 into normal fibroblasts (Guerrero et al., 2000), where it was concluded that the oncogenic versus the wild-type form of Kras is sufficient to induce a sphere-like morphology. Here, we show that the variation in expression level of the constitutively active KrasV12 mutation is sufficient to drive shifts in cell morphology rather than the mutation status alone. Our work adds to a large body of literature describing the effect of oncogenic Ras transformation on signaling and motility (Sahai et al., 2001, Lee and Helfman, 2004, Pawlak and Helfman, 2002). However, the past studies have compared the effect of wild-type versus mutated protein. Here, we show in a lung cancer model the relevance for differential expression of the mutated protein. We were able to dissect the signaling pathway in two independent cellular in vitro model systems. We could also show that such signaling is differentially activated in human lung tumor sections indicating the clinical relevance of our findings.
We show that KrasV12 protein levels respond to prototypical environmental cues in malignant solid tumors such as hypoxia and ECM stiffness. Under low Oxygen conditions, KrasV12 levels increase overall, driving a tumorigenic program of sphere-like, non-adherent cells (supplementary figure 5). In contact with an increasingly stiffer environment KrasV12 levels are suppressed, triggering the self-polarization and spreading of highly motile cells. Our data showed that low KrasV12 cells gain gradually a more sphere-like morphology on softer substrates with a range of 28, 15 or 1.5 kPa (supplementary figure 4 (a)). KrasV12 levels get increased on soft substrates with a stiffness of 15 or 1.5 kPa but not on 28 kPa stiff surfaces (figure 7 (c), supplementary figure 4 (d)). This data could indicate a threshold between 28 and 15 kPa for the mechano-sensitive response in KrasV12 protein levels. Much additional work will be required to define the exact mechanical conditions and mechanisms for this expression switch. Similarly, the link between hypoxia and KrasV12 protein levels remains to be discovered. Here, we focused on the pathway that relates KrasV12, expression to a morphological switch between a sphere-like, immotile and a spread, motile cell phenotype. Our data establish that the switch is conferred by a mechano-sensitive double-negative feedback between KrasV12 expression and FAK/ERK/Myosin II activation (supplementary figure 5). Interference with the pathway at any level led to a conversion of the cellular state in terms of KrasV12 expression, signaling, and the morphology and motility features. Reduction in ERK as well as Myosin II activity induced an adherent and motile phenotype with reduced KrasV12 levels and FAK activation. Consistent with this, reduction of FAK phosphorylation switched the phenotype from adherent to a sphere-like morphology with high KrasV12 levels and increased ERK activity. Complementary to these findings, ECM stiffness modulation led to the same changes with increased KrasV12 levels and ERK activity, sphere-like morphology and reduced FAK activation. All these data demonstrate the robustness and consistency of the pathway. Critically, at this point we have no reasons to believe that the observed morphological switch represents a transition between an amoeboid and a mesenchymal state as described, for example, for melanoma and fibrosarcoma cell lines (Sanz-Moreno et al., 2008, Wolf et al., 2003). Amoeboid cells lack adhesion engagement. In our cell models, however, sphere-like cells can engage with the ECM both in 2D and 3D. We also have no evidence that the sphere-like cell state develops a low-adhesion mode of motility in constrained 3D environments as is typical for amoeboid cells (Liu et al., 2015). Therefore, given our results of a direct relation between KrasV12 levels and Myosin II activity, we rather suggest that the observed switch in morphology and motility relates to epithelial cell states with high and low contractility, as described for both 2D and 3D scenarios in (Lomakin et al., 2015). Indeed, we were able to trigger conversion between the sphere-like and spread cell state by down- and up-regulation of Myosin II activity.
In support of our proposal of a switch-like behavior of the pathway, we were able to identify mainly two populations with distinct morphologies and KrasV12 levels, first by mechanical separation from the same culture (figure 1(c), (h)) and second by isolation of stable clones (figure 3(a)–(c)). Moreover, by flow cytometry of a heterogeneous population of patient-derived lung cancer cells harboring intrinsically the KrasV12 mutation we found a separation between low and high KrasV12-expressing cells, and a small population of very high KrasV12 cells (supplementary figure 4(h)). Previous studies found Kras associated in a complex together with unligated integrins located within the plasma membrane driving tumorigenicity (Seguin et al., 2014). Our studies suggest that upon adhesion engagement as measured by FAK activation KrasV12 gets down-regulated. Together, these two studies put Kras within a pathway where adhesions can drive changes in Kras signaling and thus regulate malignant cellular behavior. The observed switch-like transition occurs over a period of less than 24 h. Of note, this time scale matches the time scale of KrasV12 protein half-life (Shukla et al., 2014), suggesting that protein degradation may be the rate-limiting factor in transitioning between the two cellular states and leading us to speculate that the regulatory effectors downstream of hypoxia and adhesion stimuli converge onto proteolytic pathways. We cannot completely exclude the possibility that the hypoxia-induced upregulation of KrasV12 could also be driven by a clonal selection. However, the upregulation of KrasV12 in a low attachment environment as well as the reverse downregulation by induction of attachment both occur on a time scale of hours or days, which seems to rule out a clonal selection mechanism. Future work will be necessary to unravel the potentially very complex relations between hypoxia, adhesion signaling and KrasV12.
Our findings also present evidence that the KrasV12-expression switch facilitates the formation of cell collectives composed of a highly mobile and a highly tumorigenic unit, in which highly mobile cells can efficiently disseminate highly tumorigenic cells. At this point we can only speculate that the two states are driven by adhesion signaling and differences in Myosin II activity in a 3D environment. Yet, it is intriguing to note recent reports that show invasive cell clusters and multi-cellular aggregates of circulating tumor cells correlate with the worst outcome for cancer patients (Aceto et al., 2014). Our in vitro experiments provide a new potential mechanism of how such clusters can form in KrasV12 mutated cancers. More detailed analysis needs to be performed in future studies to dissect the nature and role of such collective clusters.
Supplementary Material
Supplementary figure 1 - Effect of hypoxia on KrasV12 expression but not transcription.
(a), KrasV12 protein increase in HBECp53-cMyc-Kras and H2887 cultured in 1% O2 for 6 weeks compared to culture in 21% O2. (b), Same samples as in (a) were also analyzed for total (wild-type and mutated) Kras mRNA levels showing no increase in mRNA levels upon long-term hypoxia as measured with digital droplet PCR. mRNA levels of each cell line were normalized to levels measured in 21%. (n=5, Error bars show standard deviation). (c), cMyc expression is unaffected by long-term hypoxia in HBEC p53-cMyc-Kras and H2887. (d), p53 expression is unaffected by long-term hypoxia in HBEC p53-cMyc-Kras and H2887. (e), Consecutive lung tumor sections tested positive for KrasV12 mutation were stained for Kras and Hif1α as a marker for hypoxic areas. Note the overlay of Kras positive staining and hypoxic areas. Scale bars: 500 μm.
Supplementary figure 2 - Cellular morphology correlates with levels of KrasV12 expression in vivo.
(a), Lung tumor sections stained with Kras show heterogeneous KrasV12 expression levels throughout the tumor. Scale bar: 500 μm. (b), Areas of low KrasV12 expression were defined visually by low brown stain (outlined area). (c), Hotspots of high KrasV12 expression were identified visually by strong brown staining (circled areas). (d), Illustration of nuclei shape analysis in low and high KrasV12 areas shown for one example from patient #1. Scale bar: 50 μm. (e), Examples of low and high KrasV12 expression areas for all patients are shown. Scale bar: 50 μm. (f), Statistical analysis of nuclei’s aspect ratio measurements from low and high KrasV12 tumor areas analyzed in 5 individual patients show significant decrease. (***: P < 0.001 by Mann-Whitney test). This demonstrates a more round, sphere-like cellular shape of cancer cells with high KrasV12 expression in vivo. Error bars show standard deviation.
Supplementary figure 3 – Quantification of migration behavior depending on KrasV12 levels, differential activation of FAK/ERK/Myosin II pathway and conversion of clonal phenotype upon pathway interference.
(a), Western blot analysis confirming the specificity of mutant RasV12 antibody. No signal is detected in HBEC bearing only wild-type Kras or in lung cancer cells with KrasC12 mutation (HCC4017). (b), Adherent and sphere-like clones isolated form HBECp53-cMyc-Kras show same levels of cMyc expression. (c), Migration mode was analyzed of single, adherent cells from low (n=50) and high KrasV12 clones (n=39) imaged over a period of 6 h. Persistent movement was defined as no stopping time observed during tracking, accordingly interrupted movement as at least one stopping period where the cell is not actively moving forward. All analyzed cells were categorized and percentage of migration behavior was plotted. Low KrasV12 cells mostly migrate very persistently whereas high KrasV12 cells show interrupted movement. (d), Same analysis was performed in low KrasV12 cells infected with control empty GFP vector (n=59) or GFPKrasV12 (n=60). Upon over-expression of GFPKrasV12 more cells switch to interrupted migration behavior as observed in high KrasV12 clones. (e), Western blot analysis of separated adherent and spheres-like cells from H2887 cell culture confirmed reduced FAK activation, high ERK and high Myosin activity in the spheres compared to the adherent population. Compare phosphorylated FAK (pFAK) to total FAK (FAK); phosphorylated ERK (pERK) to total ERK (ERK); phosphorylated MLC (pMLC) to total MLC (MLC). Upregulation of KrasV12 in the spheres was confirmed in figure 1(h). (f), Quantification of migration behavior in control (n=50) or ERK inhibited (ERK inh., n=40) cells shows increase in persistent migrating cells upon ERK inh. (g), Quantification of migration behavior in control (n=74) or Myosin inhibited (Myo. inh., n=80) cells shows increase in persistent migrating cells upon Myo. inh. (h), Activation of Myosin using CalyculinA (CalA) converts low KrasV12 adherent into sphere-like clones. Scale bar, 100 μm. (i), Such treatment leads to increase in KrasV12 levels. (*: P < 0.05 by ANOVA-test, ±: standard error). n indicates number of data points. (j), FAK signaling was analyzed by western blot and ratio analysis of phosphorylated FAK (pFAK) and total FAK (FAK) in CalA and control cells. (±: standard error). n indicates number of data points. (k), Activation of Myosin led to increase in ERK activation measured by western blot and ratio analysis of phosphorylated ERK (pERK) to total ERK (ERK). (#x0002A;*: P < 0.01 by Mann-Whitney test, ±: standard error). n indicates number of data points. (b)-(k) All experiments were performed in 2% O2.
Supplementary figure 4 - KrasV12-expression in micro-environments of variable stiffness.
(a), Quantification of cellular morphologies were analyzed in low KrasV12 clones in response to different substrate stiffness. Error bars indicate standard deviation. ***: P < 0.001 by ANOVA-test. (n=6). (b), Low KrasV12 cells were seeded for 48 h on infinite stiff plastic or on soft, elastically supported surfaces with a stiffness of 15 or 1.5 kPa to modulate cellular mechanical stimulation and protein extracts were analyzed for FAK signaling by western blot and ratio analysis of phosphorylated FAK (pFAK) and total FAK (FAK). Decrease of cellular mechanical tension by culture on soft substrates decreased FAK signaling. Error bars indicate standard error. ***: P < 0.001 by ANOVA-test. (n=3). (c), At the same time ERK activation increased on soft substrates measured by western blot and ratio analysis of phosphorylated ERK (pERK) to total ERK (ERK). Error bars indicate standard error. *: P < 0.05 by ANOVA-test. (n=3). (d), No increase of KrasV12 levels was observed in low KrasV12 cells seeded on 28 kPa stiff substrates. (e), Increase in Kras expression levels, as well as FAK signaling reduction (ratio of phosphorylated FAK (pFAK) and total FAK (FAK)) and ERK activation (ratio of phosphorylated ERK (pERK) and total ERK (ERK)) after 24 h on soft, elastic surfaces (15 or 1.5 kPa) was confirmed in H2887. Substrates were all coated equally with Gelatin. (f), Change in morphology on soft, elastic surfaces (15 or 1.5 kPa) to more sphere-like cells compared to stiff plastic dishes was confirmed using H2887. Scale bar = 100 μm. (g), Quantification of cellular morphologies confirmed this observation. Error bars indicate standard deviation. ***: P < 0.001 by ANOVA-test. (n=6). (h), High KrasV12 cells were seeded as single cells on micro-patterned chips and spread for 1 h before analyzing cell morphologies. Cells scored as adherent are marked in red and sphere-like ones in blue. (i), Cell morphologies were quantified in low and high KrasV12 clones on micro-patterned chips coated with 5, 20, 100 or 200 μg/ml Collagen I. Low KrasV12 clones give rise to significantly more adherent cells compared to high KrasV12 ones regardless of the coating density. ***: P < 0.001 by Student’s t-test. Error bars show standard error. (n=11 to 16). Scale bar = 50 μm. (j), KrasV12 intensities were analyzed by flow cytometry in H2887 and revealed distinct populations of low and high KrasV12 cells (black) compared to only secondary antibody control (gray). (a)-(j) All experiments were performed in 2% O2.
Supplementary figure 5 - Schematic summary of main findings.
Differential levels of KrasV12 initiate two cellular states. Hypoxia-mediated high KrasV12 levels leads to a sphere-like, non-motile phenotype via activation of the pERK/pMLC (Myosin light chain). In this state FAK phosphorylation is low, but via ECM signaling (stiffness or adhesion) FAK gets phosphorylated, which leads to low levels of KrasV12 and subsequently to less pERK and pMLC activity. This initiates an adherent and highly mobile phenotype. Reduction in adhesion-dependent mechano-stimulation can in turn convert this state back into high KrasV12 spheres. Both states were found to be able to move together as a functional unit of highly mobile low KrasV12 and tumorigenic high KrasV12 cells.
Movie1: Fast cell migration of low KrasV12 clone. Low KrasV12 cells were imaged for 6 h with phase contrast in 2% O2. Cells spread out and show a fast and persistent migration behavior. Overview of cells imaged in phase contrast (left), examples of migration trajectories (middle), overlay zoom of phase contrast and migration trajectories (right). Zoomed area is indicated with the white box. Images were acquired every 10 min. Display rate is 6 frames per second. Scale bar, 100 μm.
Movie2: Slow cell migration of high KrasV12 clone. High KrasV12 cells were imaged for 6 h with phase contrast in 2% O2. Some cells spread out and show a slow and interrupted migration behavior. Overview of cells imaged in phase contrast (left), examples of migration trajectories (middle), overlay zoom of phase contrast and migration trajectories (right). Floating cells or debris occurs due to detachment of spheres from the dish and therefore due to the cell culture technique to maintain floating spheres in culture. Zoomed area is indicated with the white box. Images were acquired every 10 min. Display rate is 6 frames per second. Scale bar, 100 μm.
Movie3: Membrane localization of GFPKrasV12. Low KrasV12 clone was transduced with GFPKrasV12 and membrane localization was confirmed using light sheet fluorescence microscopy. The movie shows a 3D volume rendering of the cell, followed by an animation of moving dual ortho-slices. One ortho-slice always shows a plane parallel to the substrate, whereas the other always shows a plane perpendicular to the substrate. Scale bar, 10 μm in the first image.
Movie4: Fast cell migration of GFP-control low KrasV12 clone. Low KrasV12 cells were transduced with empty GFP vector as control and imaged for 6 h with phase contrast and fluorescence in 2% O2. Cells spread out and show a fast and persistent migration behavior. Overview of cells imaged in phase contrast and fluorescence (left), examples of migration trajectories (middle), overlay zoom of phase contrast and migration trajectories (right). Zoomed area is indicated with the white box. Images were acquired every 10 min. Display rate is 6 frames per second. Scale bar, 100 μm.
Movie5: Conversion of cell migration of GFPKrasV12-transduced low KrasV12 clone. Low KrasV12 cells were transduced with GFPKrasV12 vector and imaged for 6 h with phase contrast and fluorescence in 2% O2. Cells form clusters of high GFPKrasV12 expression (compare Movie4). Some cells spread out and show a slower and interrupted migration behavior. Overview of cells imaged in phase contrast and fluorescence (left), examples of migration trajectories (middle), overlay zoom of phase contrast and migration trajectories (right). Zoomed area is indicated with the white box. Images were acquired every 10 min. Display rate is 6 frames per second. Scale bar, 100 μm.
Movie6: Correlation of cellular morphology with GFPKrasV12 levels. Low KrasV12 clone was transduced with GFPKrasV12 and a z-stack was acquired using light sheet fluorescence microscopy. The movie shows a rotation of a 3D volume rendering that starts from a side view, flips to a top view, and then flips to a side view again. Actin is shown in gray and GFPKrasV12 is shown in red. Cells with high GFPKrasV12 signal appear round, whereas cells with low signal appear adherent and spread. Intensities were adjusted to accurately display high KrasV12 signals. Due to this GFP signal in low KrasV12 cells appears almost as zero. Scale bar, 10 μm in the first image.
Movie7: Cell migration of DMSO-control high KrasV12 clone for ERK inhibition. High KrasV12 cells were treated with DMSO as control and imaged for 4 h with phase contrast in 2% O2. Some cells spread out and show a slow and interrupted migration behavior. Overview of cells imaged in phase contrast (left), examples of migration trajectories (middle), overlay zoom of phase contrast and migration trajectories (right). Zoomed area is indicated with the white box. Images were acquired every 10 min. Display rate is 6 frames per second. Scale bar, 100 μm.
Movie8: Cell migration of high KrasV12 clone after ERK inhibition. High KrasV12 cells were treated with an ERK-specific inhibitor and imaged for 4 h with phase contrast in 2% O2. Cells become more adherent and show a faster and more persistent migration behavior (compare Movie7). Overview of cells imaged in phase contrast (left), examples of migration trajectories (middle), overlay zoom of phase contrast and migration trajectories (right). Zoomed area is indicated with the white box. Images were acquired every 10 min. Display rate is 6 frames per second. Scale bar, 100 μm.
Movie9: Cell migration of DMSO-control high KrasV12 clone for Myosin II inhibition. High KrasV12 cells were treated with DMSO as control and imaged for 6 h with phase contrast in 2% O2. Some cells spread out and show a slow and interrupted migration behavior. Overview of cells imaged in phase contrast (left), examples of migration trajectories (middle), overlay zoom of phase contrast and migration trajectories (right). Zoomed area is indicated with the white box. Images were acquired every 10 min. Display rate is 6 frames per second. Scale bar, 100 μm.
Movie10: Cell migration of high KrasV12 clone after Myosin II inhibition. High KrasV12 cells were treated with blebbistatin to inhibit Myosin II-activity and imaged for 6 h with phase contrast in 2% O2. Cells become more adherent and show a faster and more persistent migration behavior (compare Movie9). Overview of cells imaged in phase contrast (left), examples of migration trajectories (middle), overlay zoom of phase contrast and migration trajectories (right). Zoomed area is indicated with the white box. Images were acquired every 10 min. Display rate is 6 frames per second. Scale bar, 100 μm.
Movie11: Cell morphology and migration on stiff, plastic substrates. Low KrasV12 cells were seeded on stiff, plastic dishes for 24 h and then imaged with phase contrast in 2% O2. Cells spread out and migrate faster compared to on elastically supported surfaces (see Movie12, 13). Overview of cells imaged in phase contrast (left), examples of migration trajectories (middle), overlay zoom of phase contrast and migration trajectories (right). Images were acquired every 10 min. Display rate is 6 frames per second. Scale bar, 100 μm.
Movie12: Cell clustering and decrease of migration on 15 kPa soft substrates. Low KrasV12 cells were seeded on 15 kPa soft elastically supported surfaces for 24 h and then imaged with phase contrast in 2% O2. Cells become smaller, form clusters and migrate slower compared to on plastic (see Movie11). Overview of cells imaged in phase contrast (left), examples of migration trajectories (middle), overlay zoom of phase contrast and migration trajectories (right). Images were acquired every 10 min. Display rate is 6 frames per second. Scale bar, 100 μm.
Movie13: Cell clustering and decrease of migration on 1.5 kPa soft substrates. Low KrasV12 cells were seeded on 1.5 kPa soft elastically supported surfaces for 24 h and then imaged with phase contrast in 2% O2. Cells become smaller, form clusters and migrate slower compared to on plastic (see Movie11). Overview of cells imaged in phase contrast (left), examples of migration trajectories (middle), overlay zoom of phase contrast and migration trajectories (right). Images were acquired every 10 min. Display rate is 6 frames per second. Scale bar, 100 μm.
Movie14: Dissemination of cell units from spheres. Live-cell imaging of dissemination of cells from spheres transferred onto attachment dish reveals units of motile and non-motile cells leaving the sphere imaged by phase contrast in 2% O2. Images are shown every 30 min. and display rate is 5 frames per second.
Movie15: Frequent migration of cell clusters. Combined phase contrast images of HBECp53-cMyc-Kras show frequent migration of multi-functional cell clusters. HBECp53-cMyc-Kras were transduced with GFPKrasV12 before imaging. Images were acquired every 10 min. Display rate is 6 frames per seconds. Scale bar, 100 μm.
Movie16: Migration of cell clusters as multi-functional unit. Phase contrast imaging shows a cell cluster consisting of one migratory and several non-motile, sphere-like cells migrate thru a 3D collagen matrix. Images were acquired every 10 min. Display rate is 4 frames per second. Scale bar, 50 μm.
Acknowledgments
Tissue samples were provided by the Cooperative Human Tissue Network, which is funded by the National Cancer Institute. Other investigators may have received specimens from the same subjects. This project was funded by the Cancer Prevention and Research Institute of Texas (R1225 to G.D.), by the National Institutes of Health (F32-GM116370 to M.D.) and by the National Institutes of Health, Pharmacological Sciences Training Grant (GM007062 to A.M.). We thank N. Loof and the Moody Foundation Flow Cytometry Facility in CRI for flow cytometry. We thank John Minna (UT Southwestern) for the lung cancer cell line H2887, Krishna Luitel for help with IHC staining and Kevin Dean for assistance with imaging and movie preparations.
References
- ACERBI I, CASSEREAU L, DEAN I, SHI Q, AU A, PARK C, CHEN YY, LIPHARDT J, HWANG ES, WEAVER VM. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr Biol (Camb) 2015 doi: 10.1039/c5ib00040h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ACETO N, BARDIA A, MIYAMOTO DT, DONALDSON MC, WITTNER BS, SPENCER JA, YU M, PELY A, ENGSTROM A, ZHU H, BRANNIGAN BW, KAPUR R, STOTT SL, SHIODA T, RAMASWAMY S, TING DT, LIN CP, TONER M, HABER DA, MAHESWARAN S. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158:1110–22. doi: 10.1016/j.cell.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APARICIO LA, BLANCO M, CASTOSA R, CONCHA A, VALLADARES M, CALVO L, FIGUEROA A. Clinical implications of epithelial cell plasticity in cancer progression. Cancer Lett. 2015 doi: 10.1016/j.canlet.2015.06.007. [DOI] [PubMed] [Google Scholar]
- AXELSON H, FREDLUND E, OVENBERGER M, LANDBERG G, PAHLMAN S. Hypoxia-induced dedifferentiation of tumor cells--a mechanism behind heterogeneity and aggressiveness of solid tumors. Semin Cell Dev Biol. 2005;16:554–63. doi: 10.1016/j.semcdb.2005.03.007. [DOI] [PubMed] [Google Scholar]
- BOETTNER B, VAN AELST L. The role of Rho GTPases in disease development. Gene. 2002;286:155–74. doi: 10.1016/s0378-1119(02)00426-2. [DOI] [PubMed] [Google Scholar]
- BRAHMBHATT AA, KLEMKE RL. ERK and RhoA differentially regulate pseudopodia growth and retraction during chemotaxis. J Biol Chem. 2003;278:13016–25. doi: 10.1074/jbc.M211873200. [DOI] [PubMed] [Google Scholar]
- CHAN CE, ODDE DJ. Traction dynamics of filopodia on compliant substrates. Science. 2008;322:1687–91. doi: 10.1126/science.1163595. [DOI] [PubMed] [Google Scholar]
- CHARRAS G, SAHAI E. Physical influences of the extracellular environment on cell migration. Nature Reviews Molecular Cell Biology. 2014;15:813–824. doi: 10.1038/nrm3897. [DOI] [PubMed] [Google Scholar]
- CROCKER JC, GRIER DG. Methods of digital video microscopy for colloidal studies. Journal of Colloid and Interface Science. 1996;179:298–310. [Google Scholar]
- DEAN KM, ROUDOT P, WELF ES, DANUSER G, FIOLKA R. Deconvolution-free Subcellular Imaging with Axially Swept Light Sheet Microscopy. Biophys J. 2015;108:2807–15. doi: 10.1016/j.bpj.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISCHER DE, JANMEY P, WANG YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–43. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- FRIEDL P. Prespecification and plasticity: shifting mechanisms of cell migration. Current Opinion in Cell Biology. 2004;16:14–23. doi: 10.1016/j.ceb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- FRIEDL P, LOCKER J, SAHAI E, SEGALL JE. Classifying collective cancer cell invasion. Nat Cell Biol. 2012;14:777–83. doi: 10.1038/ncb2548. [DOI] [PubMed] [Google Scholar]
- GILKES DM, SEMENZA GL, WIRTZ D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer. 2014;14:430–9. doi: 10.1038/nrc3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLUBOVSKAYA VM, NYBERG C, ZHENG M, KWEH F, MAGIS A, OSTROV D, CANCE WG. A small molecule inhibitor, 1,2,4,5-benzenetetraamine tetrahydrochloride, targeting the y397 site of focal adhesion kinase decreases tumor growth. J Med Chem. 2008;51:7405–16. doi: 10.1021/jm800483v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUERRERO S, CASANOVA I, FARRE L, MAZO A, CAPELLA G, MANGUES R. K-ras codon 12 mutation induces higher level of resistance to apoptosis and predisposition to anchorage-independent growth than codon 13 mutation or proto-oncogene overexpression. Cancer Res. 2000;60:6750–6. [PubMed] [Google Scholar]
- HANAHAN D, WEINBERG RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- HARRIS AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- HIRATA E, GIROTTI MR, VIROS A, HOOPER S, SPENCER-DENE B, MATSUDA M, LARKIN J, MARAIS R, SAHAI E. Intravital imaging reveals how BRAF inhibition generates drug-tolerant microenvironments with high integrin beta1/FAK signaling. Cancer Cell. 2015;27:574–88. doi: 10.1016/j.ccell.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IHLE NT, BYERS LA, KIM ES, SAINTIGNY P, LEE JJ, BLUMENSCHEIN GR, TSAO A, LIU S, LARSEN JE, WANG J, DIAO L, COOMBES KR, CHEN L, ZHANG S, ABDELMELEK MF, TANG X, PAPADIMITRAKOPOULOU V, MINNA JD, LIPPMAN SM, HONG WK, HERBST RS, WISTUBA II, HEYMACH JV, POWIS G. Effect of KRAS oncogene substitutions on protein behavior: implications for signaling and clinical outcome. J Natl Cancer Inst. 2012;104:228–39. doi: 10.1093/jnci/djr523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IRSHAD H, VEILLARD A, ROUX L, RACOCEANU D. Methods for nuclei detection, segmentation, and classification in digital histopathology: a review-current status and future potential. IEEE Rev Biomed Eng. 2014;7:97–114. doi: 10.1109/RBME.2013.2295804. [DOI] [PubMed] [Google Scholar]
- KEITH B, SIMON MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–72. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEMKE RL, CAI S, GIANNINI AL, GALLAGHER PJ, DE LANEROLLE P, CHERESH DA. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481–92. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE S, HELFMAN DM. Cytoplasmic p21Cip1 is involved in Ras-induced inhibition of the ROCK/LIMK/cofilin pathway. J Biol Chem. 2004;279:1885–91. doi: 10.1074/jbc.M306968200. [DOI] [PubMed] [Google Scholar]
- LEVENTAL KR, YU H, KASS L, LAKINS JN, EGEBLAD M, ERLER JT, FONG SF, CSISZAR K, GIACCIA A, WENINGER W, YAMAUCHI M, GASSER DL, WEAVER VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU YJ, LE BERRE M, LAUTENSCHLAEGER F, MAIURI P, CALLAN-JONES A, HEUZE M, TAKAKI T, VOITURIEZ R, PIEL M. Confinement and low adhesion induce fast amoeboid migration of slow mesenchymal cells. Cell. 2015;160:659–72. doi: 10.1016/j.cell.2015.01.007. [DOI] [PubMed] [Google Scholar]
- LO CM, WANG HB, DEMBO M, WANG YL. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79:144–52. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOMAKIN AJ, LEE KC, HAN SJ, BUI DA, DAVIDSON M, MOGILNER A, DANUSER G. Competition for actin between two distinct F-actin networks defines a bistable switch for cell polarization. Nat Cell Biol. 2015 doi: 10.1038/ncb3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALLIRI A, COLLARD JG. Role of Rho-family proteins in cell adhesion and cancer. Curr Opin Cell Biol. 2003;15:583–9. doi: 10.1016/s0955-0674(03)00098-x. [DOI] [PubMed] [Google Scholar]
- MENDOZA MC, ER EE, BLENIS J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends in Biochemical Sciences. 2011a;36:320–328. doi: 10.1016/j.tibs.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENDOZA MC, ER EE, ZHANG W, BALLIF BA, ELLIOTT HL, DANUSER G, BLENIS J. ERK-MAPK drives lamellipodia protrusion by activating the WAVE2 regulatory complex. Mol Cell. 2011b;41:661–71. doi: 10.1016/j.molcel.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENDOZA MC, VILELA M, JUAREZ JE, BLENIS J, DANUSER G. ERK reinforces actin polymerization to power persistent edge protrusion during motility. Science Signaling. 2015:8. doi: 10.1126/scisignal.aaa8859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITRA SK, HANSON DA, SCHLAEPFER DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- MORRIS EJ, JHA S, RESTAINO CR, DAYANANTH P, ZHU H, COOPER A, CARR D, DENG Y, JIN W, BLACK S, LONG B, LIU J, DINUNZIO E, WINDSOR W, ZHANG R, ZHAO S, ANGAGAW MH, PINHEIRO EM, DESAI J, XIAO L, SHIPPS G, HRUZA A, WANG J, KELLY J, PALIWAL S, GAO X, BABU BS, ZHU L, DAUBLAIN P, ZHANG L, LUTTERBACH BA, PELLETIER MR, PHILIPPAR U, SILIPHAIVANH P, WITTER D, KIRSCHMEIER P, BISHOP WR, HICKLIN D, GILLILAND DG, JAYARAMAN L, ZAWEL L, FAWELL S, SAMATAR AA. Discovery of a novel ERK inhibitor with activity in models of acquired resistance to BRAF and MEK inhibitors. Cancer Discov. 2013;3:742–50. doi: 10.1158/2159-8290.CD-13-0070. [DOI] [PubMed] [Google Scholar]
- PAWLAK G, HELFMAN DM. Post-transcriptional down-regulation of ROCKI/Rho-kinase through an MEK-dependent pathway leads to cytoskeleton disruption in Ras-transformed fibroblasts. Mol Biol Cell. 2002;13:336–47. doi: 10.1091/mbc.01-02-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRIOR IA, LEWIS PD, MATTOS C. A comprehensive survey of Ras mutations in cancer. Cancer Res. 2012;72:2457–67. doi: 10.1158/0008-5472.CAN-11-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PYLAYEVA-GUPTA Y, GRABOCKA E, BAR-SAGI D. RAS oncogenes: weaving a tumorigenic web. Nat Rev Cancer. 2011;11:761–74. doi: 10.1038/nrc3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAJALINGAM K, SCHRECK R, RAPP UR, ALBERT S. Ras oncogenes and their downstream targets. Biochim Biophys Acta. 2007;1773:1177–95. doi: 10.1016/j.bbamcr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- ROBERTS PJ, STINCHCOMBE TE. KRAS mutation: should we test for it, and does it matter? J Clin Oncol. 2013;31:1112–21. doi: 10.1200/JCO.2012.43.0454. [DOI] [PubMed] [Google Scholar]
- SAHAI E. Mechanisms of cancer cell invasion. Current Opinion in Genetics & Development. 2005;15:87–96. doi: 10.1016/j.gde.2004.12.002. [DOI] [PubMed] [Google Scholar]
- SAHAI E, OLSON MF, MARSHALL CJ. Cross-talk between Ras and Rho signalling pathways in transformation favours proliferation and increased motility. EMBO J. 2001;20:755–66. doi: 10.1093/emboj/20.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANCHEZ-LAORDEN B, VIROS A, GIROTTI MR, PEDERSEN M, SATURNO G, ZAMBON A, NICULESCU-DUVAZ D, TURAJLIC S, HAYES A, GORE M, LARKIN J, LORIGAN P, COOK M, SPRINGER C, MARAIS R. BRAF inhibitors induce metastasis in RAS mutant or inhibitor-resistant melanoma cells by reactivating MEK and ERK signaling. Sci Signal. 2014;7:ra30. doi: 10.1126/scisignal.2004815. [DOI] [PubMed] [Google Scholar]
- SANZ-MORENO V, GADEA G, AHN J, PATERSON H, MARRA P, PINNER S, SAHAI E, MARSHALL CJ. Rac activation and inactivation control plasticity of tumor cell movement. Cell. 2008;135:510–23. doi: 10.1016/j.cell.2008.09.043. [DOI] [PubMed] [Google Scholar]
- SATO M, LARSEN JE, LEE W, SUN H, SHAMES DS, DALVI MP, RAMIREZ RD, TANG H, DIMAIO JM, GAO B, XIE Y, WISTUBA II, GAZDAR AF, SHAY JW, MINNA JD. Human lung epithelial cells progressed to malignancy through specific oncogenic manipulations. Mol Cancer Res. 2013;11:638–50. doi: 10.1158/1541-7786.MCR-12-0634-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAFER C, RYMARCZYK G, DING L, KIRBER MT, BOLOTINA VM. Role of molecular determinants of store-operated Ca(2+) entry (Orai1, phospholipase A2 group 6, and STIM1) in focal adhesion formation and cell migration. J Biol Chem. 2012;287:40745–57. doi: 10.1074/jbc.M112.407155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHEFFZEK K, AHMADIAN MR, KABSCH W, WIESMULLER L, LAUTWEIN A, SCHMITZ F, WITTINGHOFER A. The Ras-RasGAP complex: structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science. 1997;277:333–8. doi: 10.1126/science.277.5324.333. [DOI] [PubMed] [Google Scholar]
- SCHUBBERT S, SHANNON K, BOLLAG G. Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer. 2007;7:295–308. doi: 10.1038/nrc2109. [DOI] [PubMed] [Google Scholar]
- SEGUIN L, KATO S, FRANOVIC A, CAMARGO MF, LESPERANCE J, ELLIOTT KC, YEBRA M, MIELGO A, LOWY AM, HUSAIN H, CASCONE T, DIAO L, WANG J, WISTUBA II, HEYMACH JV, LIPPMAN SM, DESGROSELLIER JS, ANAND S, WEIS SM, CHERESH DA. An integrin beta3-KRAS-RalB complex drives tumour stemness and resistance to EGFR inhibition. Nat Cell Biol. 2014;16:457–68. doi: 10.1038/ncb2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHUKLA S, ALLAM US, AHSAN A, CHEN GA, KRISHNAMURTHY PM, MARSH K, RUMSCHLAG M, SHANKAR S, WHITEHEAD C, SCHIPPER M, BASRUR V, SOUTHWORTH DR, CHINNAIYAN AM, REHEMTULLA A, BEER DG, LAWRENCE TS, NYATI MK, RAY D. KRAS Protein Stability Is Regulated through SMURF2: UBCH5 Complex-Mediated beta-TrCP1 Degradation. Neoplasia. 2014;16:115-+. doi: 10.1593/neo.14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUPACK DG, CHO SY, KLEMKE RL. Molecular signaling mechanisms of cell migration and invasion. Immunol Res. 2000;21:83–8. doi: 10.1385/IR:21:2-3:83. [DOI] [PubMed] [Google Scholar]
- SULZMAIER FJ, JEAN C, SCHLAEPFER DD. FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer. 2014;14:598–610. doi: 10.1038/nrc3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAUPEL P, MAYER A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225–39. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- VIKIS H, SATO M, JAMES M, WANG DL, WANG Y, WANG M, JIA DM, LIU Y, BAILEY-WILSON JE, AMOS CI, PINNEY SM, PETERSEN GM, DE ANDRADE M, YANG P, WIEST JS, FAIN PR, SCHWARTZ AG, GAZDAR A, GABA C, ROTHSCHILD H, MANDAL D, KUPERT E, SEMINARA D, VISWANATHAN A, GOVINDAN R, MINNA J, ANDERSON MW, YOU M. EGFR-T790M is a rare lung cancer susceptibility allele with enhanced kinase activity. Cancer Research. 2007;67:4665–4670. doi: 10.1158/0008-5472.CAN-07-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLF K, MAZO I, LEUNG H, ENGELKE K, VON ANDRIAN UH, DERYUGINA EI, STRONGIN AY, BROCKER EB, FRIEDL P. Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J Cell Biol. 2003;160:267–77. doi: 10.1083/jcb.200209006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1 - Effect of hypoxia on KrasV12 expression but not transcription.
(a), KrasV12 protein increase in HBECp53-cMyc-Kras and H2887 cultured in 1% O2 for 6 weeks compared to culture in 21% O2. (b), Same samples as in (a) were also analyzed for total (wild-type and mutated) Kras mRNA levels showing no increase in mRNA levels upon long-term hypoxia as measured with digital droplet PCR. mRNA levels of each cell line were normalized to levels measured in 21%. (n=5, Error bars show standard deviation). (c), cMyc expression is unaffected by long-term hypoxia in HBEC p53-cMyc-Kras and H2887. (d), p53 expression is unaffected by long-term hypoxia in HBEC p53-cMyc-Kras and H2887. (e), Consecutive lung tumor sections tested positive for KrasV12 mutation were stained for Kras and Hif1α as a marker for hypoxic areas. Note the overlay of Kras positive staining and hypoxic areas. Scale bars: 500 μm.
Supplementary figure 2 - Cellular morphology correlates with levels of KrasV12 expression in vivo.
(a), Lung tumor sections stained with Kras show heterogeneous KrasV12 expression levels throughout the tumor. Scale bar: 500 μm. (b), Areas of low KrasV12 expression were defined visually by low brown stain (outlined area). (c), Hotspots of high KrasV12 expression were identified visually by strong brown staining (circled areas). (d), Illustration of nuclei shape analysis in low and high KrasV12 areas shown for one example from patient #1. Scale bar: 50 μm. (e), Examples of low and high KrasV12 expression areas for all patients are shown. Scale bar: 50 μm. (f), Statistical analysis of nuclei’s aspect ratio measurements from low and high KrasV12 tumor areas analyzed in 5 individual patients show significant decrease. (***: P < 0.001 by Mann-Whitney test). This demonstrates a more round, sphere-like cellular shape of cancer cells with high KrasV12 expression in vivo. Error bars show standard deviation.
Supplementary figure 3 – Quantification of migration behavior depending on KrasV12 levels, differential activation of FAK/ERK/Myosin II pathway and conversion of clonal phenotype upon pathway interference.
(a), Western blot analysis confirming the specificity of mutant RasV12 antibody. No signal is detected in HBEC bearing only wild-type Kras or in lung cancer cells with KrasC12 mutation (HCC4017). (b), Adherent and sphere-like clones isolated form HBECp53-cMyc-Kras show same levels of cMyc expression. (c), Migration mode was analyzed of single, adherent cells from low (n=50) and high KrasV12 clones (n=39) imaged over a period of 6 h. Persistent movement was defined as no stopping time observed during tracking, accordingly interrupted movement as at least one stopping period where the cell is not actively moving forward. All analyzed cells were categorized and percentage of migration behavior was plotted. Low KrasV12 cells mostly migrate very persistently whereas high KrasV12 cells show interrupted movement. (d), Same analysis was performed in low KrasV12 cells infected with control empty GFP vector (n=59) or GFPKrasV12 (n=60). Upon over-expression of GFPKrasV12 more cells switch to interrupted migration behavior as observed in high KrasV12 clones. (e), Western blot analysis of separated adherent and spheres-like cells from H2887 cell culture confirmed reduced FAK activation, high ERK and high Myosin activity in the spheres compared to the adherent population. Compare phosphorylated FAK (pFAK) to total FAK (FAK); phosphorylated ERK (pERK) to total ERK (ERK); phosphorylated MLC (pMLC) to total MLC (MLC). Upregulation of KrasV12 in the spheres was confirmed in figure 1(h). (f), Quantification of migration behavior in control (n=50) or ERK inhibited (ERK inh., n=40) cells shows increase in persistent migrating cells upon ERK inh. (g), Quantification of migration behavior in control (n=74) or Myosin inhibited (Myo. inh., n=80) cells shows increase in persistent migrating cells upon Myo. inh. (h), Activation of Myosin using CalyculinA (CalA) converts low KrasV12 adherent into sphere-like clones. Scale bar, 100 μm. (i), Such treatment leads to increase in KrasV12 levels. (*: P < 0.05 by ANOVA-test, ±: standard error). n indicates number of data points. (j), FAK signaling was analyzed by western blot and ratio analysis of phosphorylated FAK (pFAK) and total FAK (FAK) in CalA and control cells. (±: standard error). n indicates number of data points. (k), Activation of Myosin led to increase in ERK activation measured by western blot and ratio analysis of phosphorylated ERK (pERK) to total ERK (ERK). (#x0002A;*: P < 0.01 by Mann-Whitney test, ±: standard error). n indicates number of data points. (b)-(k) All experiments were performed in 2% O2.
Supplementary figure 4 - KrasV12-expression in micro-environments of variable stiffness.
(a), Quantification of cellular morphologies were analyzed in low KrasV12 clones in response to different substrate stiffness. Error bars indicate standard deviation. ***: P < 0.001 by ANOVA-test. (n=6). (b), Low KrasV12 cells were seeded for 48 h on infinite stiff plastic or on soft, elastically supported surfaces with a stiffness of 15 or 1.5 kPa to modulate cellular mechanical stimulation and protein extracts were analyzed for FAK signaling by western blot and ratio analysis of phosphorylated FAK (pFAK) and total FAK (FAK). Decrease of cellular mechanical tension by culture on soft substrates decreased FAK signaling. Error bars indicate standard error. ***: P < 0.001 by ANOVA-test. (n=3). (c), At the same time ERK activation increased on soft substrates measured by western blot and ratio analysis of phosphorylated ERK (pERK) to total ERK (ERK). Error bars indicate standard error. *: P < 0.05 by ANOVA-test. (n=3). (d), No increase of KrasV12 levels was observed in low KrasV12 cells seeded on 28 kPa stiff substrates. (e), Increase in Kras expression levels, as well as FAK signaling reduction (ratio of phosphorylated FAK (pFAK) and total FAK (FAK)) and ERK activation (ratio of phosphorylated ERK (pERK) and total ERK (ERK)) after 24 h on soft, elastic surfaces (15 or 1.5 kPa) was confirmed in H2887. Substrates were all coated equally with Gelatin. (f), Change in morphology on soft, elastic surfaces (15 or 1.5 kPa) to more sphere-like cells compared to stiff plastic dishes was confirmed using H2887. Scale bar = 100 μm. (g), Quantification of cellular morphologies confirmed this observation. Error bars indicate standard deviation. ***: P < 0.001 by ANOVA-test. (n=6). (h), High KrasV12 cells were seeded as single cells on micro-patterned chips and spread for 1 h before analyzing cell morphologies. Cells scored as adherent are marked in red and sphere-like ones in blue. (i), Cell morphologies were quantified in low and high KrasV12 clones on micro-patterned chips coated with 5, 20, 100 or 200 μg/ml Collagen I. Low KrasV12 clones give rise to significantly more adherent cells compared to high KrasV12 ones regardless of the coating density. ***: P < 0.001 by Student’s t-test. Error bars show standard error. (n=11 to 16). Scale bar = 50 μm. (j), KrasV12 intensities were analyzed by flow cytometry in H2887 and revealed distinct populations of low and high KrasV12 cells (black) compared to only secondary antibody control (gray). (a)-(j) All experiments were performed in 2% O2.
Supplementary figure 5 - Schematic summary of main findings.
Differential levels of KrasV12 initiate two cellular states. Hypoxia-mediated high KrasV12 levels leads to a sphere-like, non-motile phenotype via activation of the pERK/pMLC (Myosin light chain). In this state FAK phosphorylation is low, but via ECM signaling (stiffness or adhesion) FAK gets phosphorylated, which leads to low levels of KrasV12 and subsequently to less pERK and pMLC activity. This initiates an adherent and highly mobile phenotype. Reduction in adhesion-dependent mechano-stimulation can in turn convert this state back into high KrasV12 spheres. Both states were found to be able to move together as a functional unit of highly mobile low KrasV12 and tumorigenic high KrasV12 cells.
Movie1: Fast cell migration of low KrasV12 clone. Low KrasV12 cells were imaged for 6 h with phase contrast in 2% O2. Cells spread out and show a fast and persistent migration behavior. Overview of cells imaged in phase contrast (left), examples of migration trajectories (middle), overlay zoom of phase contrast and migration trajectories (right). Zoomed area is indicated with the white box. Images were acquired every 10 min. Display rate is 6 frames per second. Scale bar, 100 μm.
Movie2: Slow cell migration of high KrasV12 clone. High KrasV12 cells were imaged for 6 h with phase contrast in 2% O2. Some cells spread out and show a slow and interrupted migration behavior. Overview of cells imaged in phase contrast (left), examples of migration trajectories (middle), overlay zoom of phase contrast and migration trajectories (right). Floating cells or debris occurs due to detachment of spheres from the dish and therefore due to the cell culture technique to maintain floating spheres in culture. Zoomed area is indicated with the white box. Images were acquired every 10 min. Display rate is 6 frames per second. Scale bar, 100 μm.
Movie3: Membrane localization of GFPKrasV12. Low KrasV12 clone was transduced with GFPKrasV12 and membrane localization was confirmed using light sheet fluorescence microscopy. The movie shows a 3D volume rendering of the cell, followed by an animation of moving dual ortho-slices. One ortho-slice always shows a plane parallel to the substrate, whereas the other always shows a plane perpendicular to the substrate. Scale bar, 10 μm in the first image.
Movie4: Fast cell migration of GFP-control low KrasV12 clone. Low KrasV12 cells were transduced with empty GFP vector as control and imaged for 6 h with phase contrast and fluorescence in 2% O2. Cells spread out and show a fast and persistent migration behavior. Overview of cells imaged in phase contrast and fluorescence (left), examples of migration trajectories (middle), overlay zoom of phase contrast and migration trajectories (right). Zoomed area is indicated with the white box. Images were acquired every 10 min. Display rate is 6 frames per second. Scale bar, 100 μm.
Movie5: Conversion of cell migration of GFPKrasV12-transduced low KrasV12 clone. Low KrasV12 cells were transduced with GFPKrasV12 vector and imaged for 6 h with phase contrast and fluorescence in 2% O2. Cells form clusters of high GFPKrasV12 expression (compare Movie4). Some cells spread out and show a slower and interrupted migration behavior. Overview of cells imaged in phase contrast and fluorescence (left), examples of migration trajectories (middle), overlay zoom of phase contrast and migration trajectories (right). Zoomed area is indicated with the white box. Images were acquired every 10 min. Display rate is 6 frames per second. Scale bar, 100 μm.
Movie6: Correlation of cellular morphology with GFPKrasV12 levels. Low KrasV12 clone was transduced with GFPKrasV12 and a z-stack was acquired using light sheet fluorescence microscopy. The movie shows a rotation of a 3D volume rendering that starts from a side view, flips to a top view, and then flips to a side view again. Actin is shown in gray and GFPKrasV12 is shown in red. Cells with high GFPKrasV12 signal appear round, whereas cells with low signal appear adherent and spread. Intensities were adjusted to accurately display high KrasV12 signals. Due to this GFP signal in low KrasV12 cells appears almost as zero. Scale bar, 10 μm in the first image.
Movie7: Cell migration of DMSO-control high KrasV12 clone for ERK inhibition. High KrasV12 cells were treated with DMSO as control and imaged for 4 h with phase contrast in 2% O2. Some cells spread out and show a slow and interrupted migration behavior. Overview of cells imaged in phase contrast (left), examples of migration trajectories (middle), overlay zoom of phase contrast and migration trajectories (right). Zoomed area is indicated with the white box. Images were acquired every 10 min. Display rate is 6 frames per second. Scale bar, 100 μm.
Movie8: Cell migration of high KrasV12 clone after ERK inhibition. High KrasV12 cells were treated with an ERK-specific inhibitor and imaged for 4 h with phase contrast in 2% O2. Cells become more adherent and show a faster and more persistent migration behavior (compare Movie7). Overview of cells imaged in phase contrast (left), examples of migration trajectories (middle), overlay zoom of phase contrast and migration trajectories (right). Zoomed area is indicated with the white box. Images were acquired every 10 min. Display rate is 6 frames per second. Scale bar, 100 μm.
Movie9: Cell migration of DMSO-control high KrasV12 clone for Myosin II inhibition. High KrasV12 cells were treated with DMSO as control and imaged for 6 h with phase contrast in 2% O2. Some cells spread out and show a slow and interrupted migration behavior. Overview of cells imaged in phase contrast (left), examples of migration trajectories (middle), overlay zoom of phase contrast and migration trajectories (right). Zoomed area is indicated with the white box. Images were acquired every 10 min. Display rate is 6 frames per second. Scale bar, 100 μm.
Movie10: Cell migration of high KrasV12 clone after Myosin II inhibition. High KrasV12 cells were treated with blebbistatin to inhibit Myosin II-activity and imaged for 6 h with phase contrast in 2% O2. Cells become more adherent and show a faster and more persistent migration behavior (compare Movie9). Overview of cells imaged in phase contrast (left), examples of migration trajectories (middle), overlay zoom of phase contrast and migration trajectories (right). Zoomed area is indicated with the white box. Images were acquired every 10 min. Display rate is 6 frames per second. Scale bar, 100 μm.
Movie11: Cell morphology and migration on stiff, plastic substrates. Low KrasV12 cells were seeded on stiff, plastic dishes for 24 h and then imaged with phase contrast in 2% O2. Cells spread out and migrate faster compared to on elastically supported surfaces (see Movie12, 13). Overview of cells imaged in phase contrast (left), examples of migration trajectories (middle), overlay zoom of phase contrast and migration trajectories (right). Images were acquired every 10 min. Display rate is 6 frames per second. Scale bar, 100 μm.
Movie12: Cell clustering and decrease of migration on 15 kPa soft substrates. Low KrasV12 cells were seeded on 15 kPa soft elastically supported surfaces for 24 h and then imaged with phase contrast in 2% O2. Cells become smaller, form clusters and migrate slower compared to on plastic (see Movie11). Overview of cells imaged in phase contrast (left), examples of migration trajectories (middle), overlay zoom of phase contrast and migration trajectories (right). Images were acquired every 10 min. Display rate is 6 frames per second. Scale bar, 100 μm.
Movie13: Cell clustering and decrease of migration on 1.5 kPa soft substrates. Low KrasV12 cells were seeded on 1.5 kPa soft elastically supported surfaces for 24 h and then imaged with phase contrast in 2% O2. Cells become smaller, form clusters and migrate slower compared to on plastic (see Movie11). Overview of cells imaged in phase contrast (left), examples of migration trajectories (middle), overlay zoom of phase contrast and migration trajectories (right). Images were acquired every 10 min. Display rate is 6 frames per second. Scale bar, 100 μm.
Movie14: Dissemination of cell units from spheres. Live-cell imaging of dissemination of cells from spheres transferred onto attachment dish reveals units of motile and non-motile cells leaving the sphere imaged by phase contrast in 2% O2. Images are shown every 30 min. and display rate is 5 frames per second.
Movie15: Frequent migration of cell clusters. Combined phase contrast images of HBECp53-cMyc-Kras show frequent migration of multi-functional cell clusters. HBECp53-cMyc-Kras were transduced with GFPKrasV12 before imaging. Images were acquired every 10 min. Display rate is 6 frames per seconds. Scale bar, 100 μm.
Movie16: Migration of cell clusters as multi-functional unit. Phase contrast imaging shows a cell cluster consisting of one migratory and several non-motile, sphere-like cells migrate thru a 3D collagen matrix. Images were acquired every 10 min. Display rate is 4 frames per second. Scale bar, 50 μm.