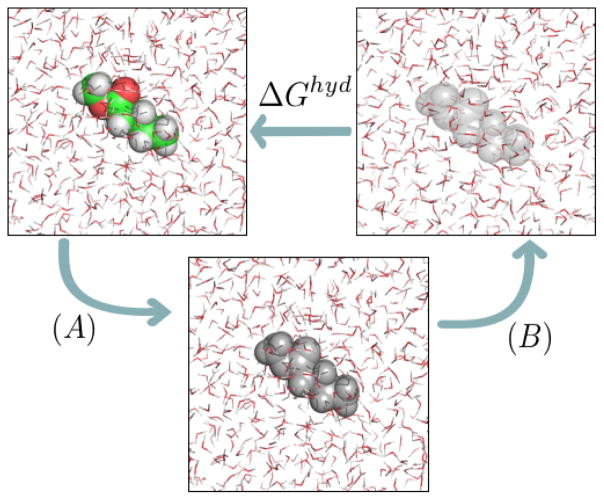
Figure 1.
Thermodynamic cycle used to calculate hydration free energies (or, more generally, solvation free energies). In (A), we have states in which charge-charge interactions between the solute and its environment are progressively turned off. In (B) dispersion interactions between solute and water are progressively turned off. Colored atoms (green for carbon, red for oxygen, white for hydrogen) have electrostatic and nonpolar interactions with the environment; gray atoms retain only nonpolar interactions; and transparent atoms have no interactions with their environment (and thus represent the solute in vacuum).