Abstract
Thyroid cancer is the most rapidly increasing cancer type among women and the second among men. Early detection greatly improves the prognosis. For this purpose, the platelet distribution width (PDW), an early indicator of platelet activation, might be useful. The aim of this study was to investigate the ability of PDW and serum albumin levels individually or in combination to distinguish between thyroid cancer and benign thyroid nodules. A total of 265 patients with thyroid cancer and 243 with benign thyroid nodules were included in a development set. Then, two groups of 130 cases were enrolled in a validation set. Patient characteristics and hematologic test data at initial diagnosis were collected. Receiver operating characteristic curves (ROC), area under the curve (AUC) values, sensitivity and specificity were estimated. Albumin levels are significantly lower and PDW significantly higher in patients with thyroid cancer compared to the benign cases. Moreover, PDW values prominently differed among three types of thyroid cancer. In addition, the combination of PDW and albumin exhibited a significantly larger AUC than either marker alone (p < 0.001). In conclusion, the combined use of PDW and albumin might be useful in distinguishing thyroid cancer from benign thyroid nodules. This promising approach could be helpful in early detection of thyroid cancer.
Keywords: Thyroid cancer, platelet distribution width, albumin, diagnosis
Introduction
Thyroid nodules are very common in the general population and are usually benign (85%-95%). During the past several decades, an increasing incidence of thyroid cancer has been reported. Although ultrasound-guided fine-needle aspiration (FNA) is currently the best established method for thyroid nodule evaluation, FNA biopsy is indeterminate in up to 30% of cases. Therefore, identification of novel diagnostic markers to distinguish thyroid cancer from benign thyroid lesions is warranted.
Activated platelets play a key role in cancer progression and metastases (Bambace and Holmes, 2011; Goubran et al., 2014). Mean platelet volume (MPV) is an indicator of activated platelets and is associated with gastric cancer, ovarian cancer, lung cancer, colon cancer, and breast cancer(Kemal et al., 2014; Kilincalp et al., 2014; Li et al., 2014; Gu et al., 2015; Kumagai et al., 2015). Platelet distribution width (PDW), another platelet parameter, indicates variation in platelet size and differentially diagnoses thrombocytopenia (Kaito et al., 2005).
Albumin is a negative acute-phase protein affected by inflammatory states. Albumin is an objective indicator of malnutrition and is a sensitive predictor of long-term outcome in patients with non-small cell lung cancer, breast cancer, ovarian cancer, advanced gastric cancer, head and neck cancer, colon and rectal carcinomas, hepatocellular carcinoma(Tateishi et al., 2005; Boonpipattanapong and Chewatanakornkul, 2006; Ataseven et al., 2015; Liu et al., 2015; Tanriverdi et al., 2015; Yamashita et al., 2015; Danan et al., 2016).
Early detection of cancers is a key aspect of cancer management because early clinical stages are easier to cure than later stages. Combination of several biomarkers for the early detection may result in enhanced sensitivities and specificities. The aim of the present study was to evaluate the ability of MPV, PDW, and albumin, individually or in combination, to distinguish between benign thyroid diseases from thyroid cancer.
Materials and Methods
Study population
The study cohort was composed of training group and validation group. We recruited a total of 768 cases from 2 different hospitals in Harbin, China. The initial training set included 265 patients with thyroid cancer and 243 patients with benign thyroid diseases who were admitted to the Third Affiliated Hospital, Harbin Medical University between January 2014 and June 2014. The validation set included 130 patients with thyroid cancer and 130 patients with benign thyroid diseases who were admitted to the Second Affiliated Hospital, Harbin Medical University between January 2014 and June 2014. The patients with benign thyroid diseases were matched for age, gender, body mass index (BMI), and smoking status. Inclusion criteria were as follows: (1) undergone complete surgical resection and diagnosis of thyroid cancer was confirmed by histology; (2) without distant metastasis at diagnosis; (3) untreated prior to blood collection. Exclusion criteria included: hematological disorders, autoimmune diseases, systemic inflammatory diseases, coronary artery disease, hypertension, diabetes mellitus, renal disease, hepatic disorder and other cancer, and medical treatment with anticoagulant, statins, and acetylic salicylic acid.
The study protocol was approved by the Ethics Committee of the Second and Third Affiliated Hospital of Harbin Medical University, Harbin, China. Written informed consents were obtained from all participants.
Clinical examination and biochemical measurements
All the subjects underwent physical examination. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Clinical data included smoking status, medical history and medication use. The whole blood samples were drawn after an 8-h overnight fasting and all samples were processed within 30 min after blood collection. White blood cell (WBC), hemoglobin, and platelet indices were determined with an autoanalyzer (Sysmex XE-2100, Kobe, Japan). The inter- and intra-assays coefficients of variation (CVs) were below 5%.
Statistical analyses
All data was expressed as means ± SD or median (interquartile range) or percentage. When the characteristics between two groups were compared, continuous variables were compared with the Student t test. When the characteristics among three groups were compared, continuous variables were compared with the one-way ANOVA. Receiver-operating characteristic curves were used to define sensitivity and specificity, and the differences in the area under the curve (AUC) were detected by using MedCalc version 13.0. A two-tailed significance threshold of 0.05 was used for all statistical tests, performed using SPSS version 22.0 (SPSS Inc., Chicago, IL, USA).
Results
The baseline characteristics of the patients are shown in Table 1. In the development set, the mean age of the patient with benign thyroid disease and thyroid cancer was 46.3 (9.8) years and 50.7 (5.1) years, respectively. Most of the patients were female and had multiple nodules. The common pathological type of benign thyroid disease and thyroid cancer are nodular goiter and papillary carcinoma, respectively. Most of the patients with thyroid cancer had low incidence of lymph node metastasis (n = 33; 12.5%) and were diagnosed as T1 (n=223; 84.2%) and stage I (n = 224; 84.5%). In the validation set, similar results were found.
Table 1.
The Characteristics of the Participants
| N (%) Development set | N (%) Validation set | |
|---|---|---|
| Benign thyroid disease | ||
| Age (years) | ||
| Median (Range) | 50 (48–53) | 51 (48-54) |
| Sex | ||
| Male | 45 (18.5) | 19 (14.6) |
| Female | 198 (81.5) | 111 (85.4) |
| Nodule | ||
| Single | 49 (20.2) | 22 (16.9) |
| Multiple | 194 (79.8) | 108 (83.1) |
| Type | ||
| Nodular goiter | 232 (95.5) | 125 (96.2) |
| Follicular adenoma | 11 (4.5) | 5 (3.8) |
| Thyroid cancer | ||
| Age (years) | ||
| Median (Range) | 46 (39–53) | 50 (42-56) |
| Sex | ||
| Male | 37 (14.0) | 116 (89.2) |
| Female | 228 (86.0) | 14 (10.8) |
| Nodule | ||
| Single | 81 (30.6) | 32 (24.6) |
| Multiple | 184 (69.4) | 98 (75.4) |
| Type | ||
| Papillary carcinoma | 152 (57.3) | 106 (81.5) |
| Follicular carcinoma | 50 (18.9) | 13 (10.0) |
| Medullary carcinoma | 63 (23.8) | 11 (8.5) |
| Tumor size | ||
| T1 | 223 (84.2) | 117 (90.0) |
| T2 | 34 (12.8) | 11 (8.5) |
| T3 | 8 (3.0) | 2 (1.5) |
| Nodal status | ||
| Positive | 33 (12.5) | 11 (8.5) |
| Negative | 232 (87.5) | 119 (91.5) |
| TNM stage | ||
| I | 224 (84.5) | 112 (86.2) |
| II | 16 (6.0) | 7 (5.4) |
| III | 10 (3.8) | 7 (5.4) |
| IV | 15 (5.7) | 4 (3.0) |
Albumin, MPV and PDW levels in benign thyroid disease and thyroid cancer are shown in Table 2. The levels of albumin and MPV are significantly reduced and PDW are significantly increased among the patients with thyroid cancer compared to the benign cases in both the development and validation sets (p < 0.001).
Table 2.
Comparison of Albumin, MPV and PDW Between Benign Group and Malignant Group
| Benign group | Malignant group | p-value | |
|---|---|---|---|
| Development set | |||
| Numbers | 243 | 265 | |
| Albumin (g/L) | 46.3(2.7) | 44.0(3.3) | < 0.001 |
| MPV (fL) | 9.8 (1.3) | 9.1 (1.2) | < 0.001 |
| PDW (%) | 14.7 (2.2) | 16.5 (1.4) | < 0.001 |
| Validation set | |||
| Numbers | 130 | 130 | |
| Albumin (g/L) | 46.6 (2.5) | 44.0 (3.3) | < 0.001 |
| MPV (fL) | 10.0 (1.3) | 9.2 (1.0) | < 0.001 |
| PDW (%) | 14.2 (2.3) | 16.1 (1.6) | < 0.001 |
Values are shown as mean (standard deviation). MPV, mean platelet volume; PDW, platelet distribution width.
We evaluated the levels of albumin, MPV and PDW levels in various benign thyroid disease and thyroid cancer of various pathological types (Table 3). In the development set, we found that albumin, MPV and PDW levels are not markedly different in different types of benign thyroid disease. Although MPV is not different in different types of thyroid cancer, albumin and PDW levels are prominently different in different types of thyroid cancer. In the validation set, similar results are found except the albumin levels in thyroid cancer. There is no statistically significant difference in albumin levels among different types of thyroid cancer (p = 0.074).
Table 3.
Albumin, MPV, and PDW Levels in the Different Pathological Types of Thyroid Masses
| N | Albumin (g/L) | Albumin (g/L) | PDW(%) | |
|---|---|---|---|---|
| Development set | ||||
| Benign thyroid disease | ||||
| Nodular goiter | 232 | 46.2 (2.7) | 9.8 (1.3) | 14.7 (2.2) |
| Follicular adenoma | 11 | 47.0 (1.2) | 9.7 (0.9) | 14.1 (2.5) |
| P-value | 0.072 | 0.216 | 0.398 | |
| Thyroid cancer | ||||
| Papillary carcinoma | 152 | 43.4 (2.6) | 9.1 (1.2) | 16.7 (1.1) |
| Follicular carcinoma | 50 | 44.6 (4.4) | 8.9 (1.0) | 16.5 (1.8) |
| Medullary carcinoma | 63 | 44.7 (3.6) | 9.3 (1.5) | 16.2 (1.6) |
| P-value | 0.01 | 0.216 | 0.037 | |
| Validation set | ||||
| Benign thyroid disease | ||||
| Nodular goiter | 125 | 46.6 (2.4) | 10.0 (1.3) | 14.2 (2.3) |
| Follicular adenoma | 5 | 45.2 (2.7) | 10.4 (1.1) | 13.4 (2.0) |
| P value | 0.206 | 0.494 | 0.442 | |
| Thyroid cancer | ||||
| Papillary carcinoma | 106 | 44.4 (3.2) | 9.1 (1.0) | 16.3 (1.2) |
| Follicular carcinoma | 13 | 46.2 (3.9) | 9.4 (1.0) | 15.3 (2.2) |
| Medullary carcinoma | 11 | 46.1 (2.9) | 9.1 (1.6) | 15.1 (2.9) |
| P-value | 0.074 | 0.568 | 0.009 | |
MPV, mean platelet volume; PDW, platelet distribution width.
In Table 4, the sensitivity, specificity, positive predictive value, negative predictive value, and area under curve (AUC) values are presented for MPV, PDW, albumin, the combination of albumin and MPV, the combination of albumin and PDW. To determine the predictive accuracy of each of the significant independent multivariate biomarkers based on the optimal cutoff values, we used ROC analysis to assess the AUC for single biomarkers and the combination of three (Table 4). When used to analyze benign thyroid masses versus thyroid cancer, PDW had the highest sensitivity (92.1%), but at the cost of an unsatisfactory low specificity (45.7%). Albumin had the highest specificity (88.9%) with an low sensitivity (50.9%). The specificity of PDW and the sensitivity of albumin increased when the combination of PDW and albumin were applied. Single biomarkers had AUC values ranging from 0.692 for MPV to 0.741 for albumin; the combination of MPV and albumin or the combination of PDW and albumin increased the AUC to 0.768 (p = 0.0005) and 0.824 (p < 0.0001), respectively. In addition, the combination of PDW and albumin exhibited a significantly larger AUC of 0.824 (0.788-0.856) in comparison with the combination of MPV and albumin (p = 0.0014) (see Figure 1 and Figure 2).
Table 4.
Receiver Operating Characteristic Curve Analyses Showing the Utility of Alone or Combined Markers for Differentiating of Benign Thyroid Diseases and Thyroid Cancer
| Tumor marker | Sensitivity | Specificity | PPV | NPV | AUC |
|---|---|---|---|---|---|
| Development set | |||||
| MPV (fL) | 0.668 | 0.72 | 0.722 | 0.655 | 0.692 (0.649-0.732) |
| PDW (%) | 0.921 | 0.457 | 0.649 | 0.841 | 0.740 (0.699-0.777) |
| Albumin (g/L) | 0.509 | 0.889 | 0.833 | 0.624 | 0.741 (0.701-0.779) |
| MPV+ albumin | 0.694 | 0.794 | 0.786 | 0.704 | 0.768 (0.729-0.804) |
| PDW+ albumin | 0.781 | 0.716 | 0.75 | 0.75 | 0.824 (0.788-0.856) |
| Validation set | |||||
| MPV (fL) | 0.762 | 0.669 | 0.697 | 0.737 | 0.722 (0.664-0.776) |
| PDW (%) | 0.807 | 0.562 | 0.648 | 0.745 | 0.757 (0.700-0.808) |
| Albumin (g/L) | 0.569 | 0.785 | 0.725 | 0.646 | 0.716 (0.657-0.770) |
| MPV+ albumin | 0.754 | 0.723 | 0.731 | 0.746 | 0.758 (0.701-0.809) |
| PDW+ albumin | 0.723 | 0.723 | 0.758 | 0.735 | 0.805 (0.752-0.852) |
MPV, mean platelet volume; PDW, platelet distribution width; PPV, positive predictive value; NPV, negative predictive value; AUC, area under curve.
Figure 1.
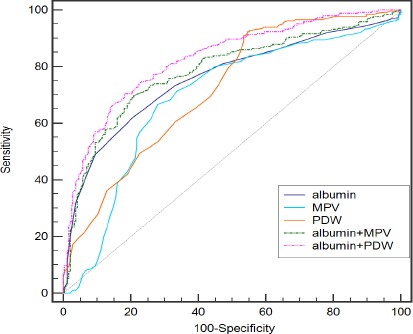
Receiver-Operator Characteristics (ROC) Curve for PDW and Albumin Combined Showing Sensitivity and 1-Specificity of the Differential Diagnosis of Thyroid Cancer and benign Thyroid Nodule in the Development Set.
Figure 2.
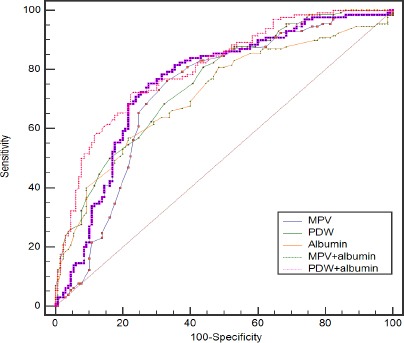
Receiver-Operator Characteristics (ROC) Curve for PDW, and Albumin Combined Showing Sensitivity and 1-Specificity of the Differential Diagnosis of Thyroid Cancer and Benign Thyroid Nodule in the Validation Set.
Discussion
Thyroid cancer is the most rapidly increasing cancer among women and the second among men. However, tumor marker evaluation in early screening is not satisfactory. In this study, we showed that the combined use of PDW and albumin can be accurately distinguished cervical cancer from benign thyroid masses.
Accumulating experimental and clinical evidences support the hypothesis that platelet activation during cancer promotes disease progression. We found that reduced MPV and increased PDW in patients with thyroid cancer. Although the mechanism is unclear, bone marrow cells (including megakaryocytes) dys-regulation may contribute to changed MPV and PDW. Platelet volume is determined both during megakaryopoiesis and during thrombopoiesis. Megakaryocytic maturation, platelet production and platelet size could be modulated by cytokines, such as interleukin-6 (IL-6), granulocytes colony stimulating factor (G-CSF) and macrophage colony stimulating factor (M-CSF)(Kaushansky, 1998). Furthermore, megakaryopoiesis and subsequent thrombopoiesis in cancer may be stimulated by the cytokines G-CSF and M-CSF, which could be secreted by tumor cells (Kowanetz et al., 2010). MPV and PDW were early indicators of activated platelets. Reduced MPV was regarded as an enhanced consumption of large platelets in inflammatory states (Kapsoritakis et al., 2001). In addition, MPV has been shown to be positively associated with levels of thrombopoietin and interleukin-6, cytokines that regulate megakaryocyte ploidy (Martin et al., 1983; Brown et al., 1997). Platelet distribution width is a measure of platelet heterogeneity. The heterogeneity in platelet volume is caused by heterogeneous demarcation of megakarocytes (Paulus, 1981). Recent new evidences suggest that thyroid stimulating hormone is an independent predictor for the diagnosis of thyroid malignancy in patients with nodular thyroid disease (Boelaert, 2009; Dorange et al., 2011; He et al., 2016). However, a cross-sectional study did not find any association between PDW and thyroid function (Ren et al., 2016). Further studies are awaited to clarify the true relationship between PDW and thyroid cancer.
Patients’ nutritional status is closely linked to cancer mortality, with one third of deaths being caused by malnutrition (Garcia-Luna et al., 2006). Serum albumin reflects the nutritional status of cancer patients and is a negative prognostic factor for survival in non-small cell lung cancer, breast cancer, ovarian cancer, advanced gastric cancer, head and neck cancer, colon and rectal carcinomas, hepatocellular carcinoma(Tateishi et al., 2005; Boonpipattanapong and Chewatanakornkul, 2006; Ataseven et al., 2015; Liu et al., 2015; Tanriverdi et al., 2015; Yamashita et al., 2015; Danan et al., 2016). In addition, albumin is a negative acute-phase protein affected by inflammatory states (Yeun and Kaysen, 1998; Al-Shaiba et al., 2004). Thus, albumin has been often investigated in the prognostic index models in patients with cancer. C-reactive protein/albumin ratio is a poor prognostic indicator in small-cell lung cancer, gastric cancer, colorectal cancer, esophageal cancer, and pancreatic cancer(Liu et al., 2015; Zhou et al., 2015; Ishizuka et al., 2016; Matsuda et al., 2016; Wu et al., 2016). Hypoalbuminemia is an objective parameter of malnutrition and PDW is an index of activated platelet. Furthermore, measurement of serum albumin and PDW is simple and cost-effective in clinical practice.
For the diagnosis of thyroid cancer, few tumor markers are highly sensitive or specific. Thyroglobulin has low specificity and sensitivity. Our study demonstrated that the AUC values for discriminating thyroid cancer patients from benign thyroid masses using this three biomarkers were 0.875 and 0.938, respectively, significantly higher than those of any single index. In addition, MPV, PDW, and albumin levels are routinely recorded in the clinical setting and can be easily estimated prior to treatment. Thus, a combination of three serum markers is a more comprehensive indicator for thyroid cancer detection than single biomarker.
In conclusion, the study showed that the combined use of MPV, PDW and albumin might be useful in the distinction of thyroid cancer and benign thyroid masses. It is necessary to validate the results and elucidate the underlying mechanism in larger cohorts.
Conflict of Interest statement
The authors declare no conflict of interest.
Acknowledgements
This work was supported financially by grants from the Harbin Medical University Cancer Hospital (JJZD2017-05).
References
- Al-Shaiba R, McMillan DC, Angerson WJ, et al. The relationship between hypoalbuminaemia, tumour volume and the systemic inflammatory response in patients with colorectal liver metastases. Br J Cancer. 2004;91:205–7. doi: 10.1038/sj.bjc.6601886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataseven B, du BA, Reinthaller A, et al. Pre-operative serum albumin is associated with post-operative complication rate and overall survival in patients with epithelial ovarian cancer undergoing cytoreductive surgery. Gynecol Oncol. 2015;138:560–5. doi: 10.1016/j.ygyno.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost. 2011;9:237–49. doi: 10.1111/j.1538-7836.2010.04131.x. [DOI] [PubMed] [Google Scholar]
- Boelaert K. The association between serum TSH concentration and thyroid cancer. Endocr Relat Cancer. 2009;16:1065–72. doi: 10.1677/ERC-09-0150. [DOI] [PubMed] [Google Scholar]
- Boonpipattanapong T, Chewatanakornkul S. Preoperative carcinoembryonic antigen and albumin in predicting survival in patients with colon and rectal carcinomas. J Clin Gastroenterol. 2006;40:592–5. doi: 10.1097/00004836-200608000-00006. [DOI] [PubMed] [Google Scholar]
- Brown AS, Hong Y, de Belder A, et al. Megakaryocyte ploidy and platelet changes in human diabetes and atherosclerosis. Arterioscler Thromb Vasc Biol. 1997;17:802–7. doi: 10.1161/01.atv.17.4.802. [DOI] [PubMed] [Google Scholar]
- Danan D, Shonka DC, Jr, Selman Y, et al. Prognostic value of albumin in patients with head and neck cancer. Laryngoscope. 2016;126:1567–71. doi: 10.1002/lary.25877. [DOI] [PubMed] [Google Scholar]
- Dorange A, Triau S, Mucci-Hennekinne S, et al. An elevated level of TSH might be predictive of differentiated thyroid cancer. Ann Endocrinol (Paris) 2011;72:513–21. doi: 10.1016/j.ando.2011.07.032. [DOI] [PubMed] [Google Scholar]
- Garcia-Luna PP, Parejo CJ, Pereira CJL. Causes and impact of hyponutrition and cachexia in the oncologic patient. Nutr Hosp. 2006;21:10–6. [PubMed] [Google Scholar]
- Goubran HA, Stakiw J, Radosevic M, et al. Platelet-cancer interactions. Semin Thromb Hemost. 2014;40:296–305. doi: 10.1055/s-0034-1370767. [DOI] [PubMed] [Google Scholar]
- Gu M, Zhai Z, Huang L, et al. Pre-treatment mean platelet volume associates with worse clinicopathologic features and prognosis of patients with invasive breast cancer. Breast Cancer. 2015;23:752–60. doi: 10.1007/s12282-015-0635-6. [DOI] [PubMed] [Google Scholar]
- He LZ, Zeng TS, Pu L, et al. Thyroid hormones, autoantibodies, ultrasonography, and clinical parameters for predicting thyroid cancer. Int J Endocrinol. 2016;2016:8215834. doi: 10.1155/2016/8215834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka M, Nagata H, Takagi K, et al. Clinical significance of the C-reactive protein to albumin ratio for survival after surgery for colorectal cancer. Ann Surg Oncol. 2016;23:900–7. doi: 10.1245/s10434-015-4948-7. [DOI] [PubMed] [Google Scholar]
- Kaito K, Otsubo H, Usui N, et al. Platelet size deviation width, platelet large cell ratio, and mean platelet volume have sufficient sensitivity and specificity in the diagnosis of immune thrombocytopenia. Br J Haematol. 2005;128:698–702. doi: 10.1111/j.1365-2141.2004.05357.x. [DOI] [PubMed] [Google Scholar]
- Kapsoritakis AN, Koukourakis MI, Sfiridaki A, et al. Mean platelet volume: a useful marker of inflammatory bowel disease activity. Am J Gastroenterol. 2001;96:776–81. doi: 10.1111/j.1572-0241.2001.03621.x. [DOI] [PubMed] [Google Scholar]
- Kaushansky K. Growth factors and hematopoietic cell fate. A new feature: controversies in hematology. Blood. 1998;92:345–4. [PubMed] [Google Scholar]
- Kemal Y, Demirag G, Ekiz K, et al. Mean platelet volume could be a useful biomarker for monitoring epithelial ovarian cancer. J Obstet Gynaecol. 2014;34:515–8. doi: 10.3109/01443615.2014.912620. [DOI] [PubMed] [Google Scholar]
- Kilincalp S, Ekiz F, Basar O, et al. Mean platelet volume could be possible biomarker in early diagnosis and monitoring of gastric cancer. Platelets. 2014;25:592–4. doi: 10.3109/09537104.2013.783689. [DOI] [PubMed] [Google Scholar]
- Kowanetz M, Wu X, Lee J, et al. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+granulocytes. Proc Natl Acad Sci U S A. 2010;107:21248–55. doi: 10.1073/pnas.1015855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai S, Tokuno J, Ueda Y, et al. Prognostic significance of preoperative mean platelet volume in resected non-small-cell lung cancer. Mol Clin Oncol. 2015;3:197–201. doi: 10.3892/mco.2014.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, Li Y, Jiang Z, et al. Elevated mean platelet volume is associated with presence of colon cancer. Asian Pac J Cancer Prev. 2014;15:10501–4. doi: 10.7314/apjcp.2014.15.23.10501. [DOI] [PubMed] [Google Scholar]
- Liu X, Meng QH, Ye Y, et al. Prognostic significance of pretreatment serum levels of albumin, LDH and total bilirubin in patients with non-metastatic breast cancer. Carcinogenesis. 2015;36:243–8. doi: 10.1093/carcin/bgu247. [DOI] [PubMed] [Google Scholar]
- Liu X, Sun X, Liu J, et al. Preoperative C-reactive protein/albumin ratio predicts prognosis of patients after curative resection for gastric cancer. Transl Oncol. 2015;8:339–45. doi: 10.1016/j.tranon.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JF, Trowbridge EA, Salmon G, et al. The biological significance of platelet volume: its relationship to bleeding time, platelet thromboxane B2 production and megakaryocyte nuclear DNA concentration. Thromb Res. 1983;32:443–60. doi: 10.1016/0049-3848(83)90255-4. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Niihara M, Tsubosa Y, et al. Clinical significance of postoperative recovery of serum albumin levels in patients with esophageal cancer who underwent transthoracic esophagectomy. Surg Today. 2016;46:1138–45. doi: 10.1007/s00595-015-1300-6. [DOI] [PubMed] [Google Scholar]
- Paulus JM. Recent advances in the story of megakaryocyte physiology. Pathol Biol (Paris) 1981;29:133–5. [PubMed] [Google Scholar]
- Ren X, Meng Z, Liu M, et al. No associations exist between mean platelet volume or platelet distribution width and thyroid function in Chinese. Medicine (Baltimore) 2016;95:e4573. doi: 10.1097/MD.0000000000004573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanriverdi O, Avci N, Oktay E, et al. Pretreatment serum albumin level is an independent prognostic factor in patients with stage IIIB non-small cell lung cancer: A study of the Turkish descriptive oncological researches group. Asian Pac J Cancer Prev. 2015;16:5971–6. doi: 10.7314/apjcp.2015.16.14.5971. [DOI] [PubMed] [Google Scholar]
- Tateishi R, Yoshida H, Shiina S, et al. Proposal of a new prognostic model for hepatocellular carcinoma: an analysis of 403 patients. Gut. 2005;54:419–25. doi: 10.1136/gut.2003.035055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Guo J, Guo L, et al. The C-reactive protein/albumin ratio predicts overall survival of patients with advanced pancreatic cancer. Tumour Biol. 2016;37:12525–33. doi: 10.1007/s13277-016-5122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita K, Ushiku H, Katada N, et al. Reduced preoperative serum albumin and absence of peritoneal dissemination may be predictive factors for long-term survival with advanced gastric cancer with positive cytology test. Eur J Surg Oncol. 2015;41:1324–32. doi: 10.1016/j.ejso.2015.05.021. [DOI] [PubMed] [Google Scholar]
- Yeun JY, Kaysen GA. Factors influencing serum albumin in dialysis patients. Am J Kidney Dis. 1998;32:S118–25. doi: 10.1016/s0272-6386(98)70174-x. [DOI] [PubMed] [Google Scholar]
- Zhou T, Zhan J, Hong S, et al. Ratio of C-reactive protein/albumin is an inflammatory prognostic score for predicting overall survival of patients with small-cell lung cancer. Sci Rep. 2015;5:10481. doi: 10.1038/srep10481. [DOI] [PMC free article] [PubMed] [Google Scholar]


