Fig. 6. Comparison of the CXCL12:CXCR4 hybrid model to the vMIP-II:CXCR4 crystal structure.
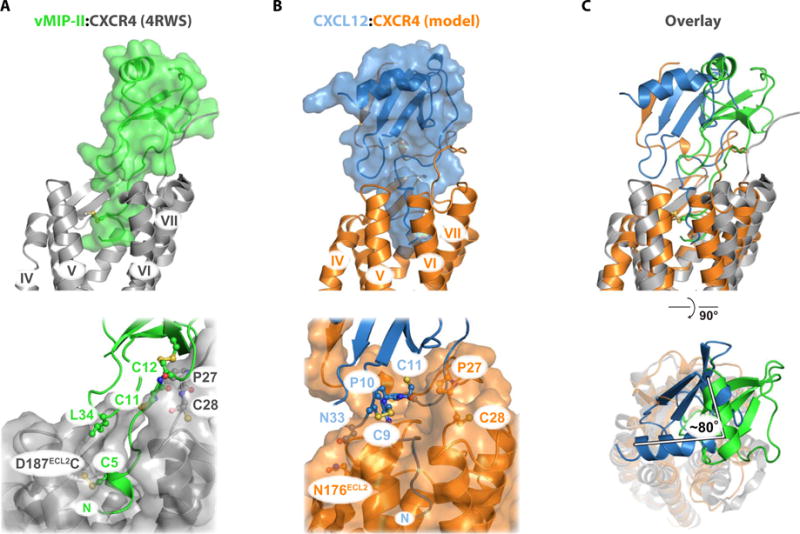
(A) vMIP-II:CXCR4 x-ray structure (PDB 4RWS). vMIP-II forms an intermolecular β strand between the CC-motif and CXCR4 residues Pro27 and Cys28, which positions the globular domain near TM1 and TM2. (B) CXCL12:CXCR4 hybrid model derived from docking the LM:CXCR41–38 NMR structure (PDB 2N55) to the CXCR4 x-ray structure (PDB 3ODU). The globular domain of the chemokine makes contacts with TM4, TM5, and TM6, making distinct site 1, 1.5, and 2 contacts relative to the vMIP-II:CXCR4 structure. (C) Pairwise alignment between CXCR4 residues 28 to 300 (1972 atoms) of the vMIP-II:CXCR4 structure and the CXCL12:CXCR4 model, yielding an RMSD of 2.2 Å.
