Abstract
IMPORTANCE
Mailed fecal immunochemical test (FIT) outreach is more effective than colonoscopy outreach for increasing 1-time colorectal cancer (CRC) screening, but long-term effectiveness may need repeat testing and timely follow-up for abnormal results.
OBJECTIVE
Compare the effectiveness of FIT outreach and colonoscopy outreach to increase completion of the CRC screening process (screening initiation and follow-up) within 3 years.
DESIGN, SETTING, AND PARTICIPANTS
Pragmatic randomized clinical trial from March 2013 to July 2016 among 5999 participants aged 50 to 64 years who were receiving primary care in Parkland Health and Hospital System and were not up to date with CRC screenings.
INTERVENTIONS
Random assignment to mailed FIT outreach (n = 2400), mailed colonoscopy outreach (n = 2400), or usual care with clinic-based screening (n = 1199). Outreach included processes to promote repeat annual testing for individuals in the FIT outreach group with normal results and completion of diagnostic and screening colonoscopy for those with an abnormal FIT result or assigned to colonoscopy outreach.
MAIN OUTCOMES AND MEASURES
Primary outcome was screening process completion, defined as adherence to colonoscopy completion, annual testing for a normal FIT result, diagnostic colonoscopy for an abnormal FIT result, or treatment evaluation if CRC was detected. Secondary outcomes included detection of any adenoma or advanced neoplasia (including CRC) and screening-related harms (including bleeding or perforation).
RESULTS
All 5999 participants (median age, 56 years; women, 61.9%) were included in the intention-to-screen analyses. Screening process completion was 38.4% in the colonoscopy outreach group, 28.0% in the FIT outreach group, and 10.7% in the usual care group. Compared with the usual care group, between-group differences for completion were higher for both outreach groups, and highest in the colonoscopy outreach group. Compared with usual care, the between-group differences in adenoma and advanced neoplasia detection rates were higher for both outreach groups, and highest in the colonoscopy outreach group. There were no screening-related harms in any groups.
| Between-Group Differences, % (95% CI) | ||||||
|---|---|---|---|---|---|---|
| Colonoscopy Outreach vs Usual Care | P Value | FIT Outreach vs Usual Care | P Value | Colonoscopy Outreach vs FIT Outreach | P Value | |
| Screening process completion | 27.7 (25.1 to 30.4) | <.001 | 17.3 (14.8 to 19.8) | <.001 | 10.4 (7.8 to 13.1) | <.001 |
| Detection rate for adenoma | 10.3 (9.5 to 12.1) | <.001 | 1.3 (−0.1 to 2.8) | .08 | 9.0 (7.3 to 10.7) | <.001 |
| Detection rate for advanced neoplasia | 3.1 (2.0 to 4.1) | <.001 | 0.7 (−0.2 to 1.6) | .13 | 2.4 (1.3 to 3.3) | <.001 |
CONCLUSIONS AND RELEVANCE
Among persons aged 50 to 64 years receiving primary care at a safety-net institution, mailed outreach invitations offering FIT or colonoscopy compared with usual care increased the proportion completing CRC screening process within 3 years. The rate of screening process completion was higher with colonoscopy than FIT outreach.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT01710215
Colorectal cancer (CRC) is the second leading cause of cancer-related death in the United States. Screening can reduce CRC incidence and mortality. The US Preventive Services Task Force (USPSTF) gives CRC screening a grade A recommendation (defined as a recommended service because there is high certainty that the net benefit is substantial) for individuals aged 50 to 75 years.1 However, effectiveness is limited by under use and suboptimal adherence to guideline-recommended follow-up, including repeat testing for normal test results and diagnostic follow-up of abnormal test results. Studies have demonstrated failures at each step in the screening process, which are associated with increased CRC mortality: 30% to 50% of patients do not initiate screening,2,3 40% to 60% of patients with normal results do not undergo repeat screening,4,5 and more than 50% of patients with abnormal results do not complete a follow-up evaluation.6,7
The best strategies to increase screening process completion are uncertain. Colonoscopy may be accepted by individuals seeking tests with high sensitivity and low frequency but not by those averse to invasive testing requiring bowel preparation, sedation, and transportation.8 Fecal immunochemical test (FIT) acceptance may be highest among individuals seeking at-home testing but lower among those averse to obtaining stool samples or performing annual testing.9 In the United States, most screening is visit-based and dependent on primary care encounters. Complementing visit-based screening with mailed outreach invitations increases 1-time screening among racially diverse and socioeconomically disadvantaged populations2; however, most studies of screening strategies have focused on single steps in the screening process, with few comparing their effect on completion of the entire process over time.10–12
One-year results of a pragmatic, randomized comparative effectiveness trial of FIT outreach, colonoscopy outreach, and usual care for increasing CRC-screening initiation in a large safety-net health system were previously reported.13 This study reports a primary outcome comparing FIT outreach, colonoscopy outreach, and usual care for increasing CRC screening process completion (screening initiation and follow-up) within a 3-year period.
Methods
Study Population
Parkland Health and Hospital System, a publicly funded integrated safety-net health system, includes a 900-bed hospital and 12 community-based primary care clinics, specialty clinics, and colonoscopy suites. Parkland offers a sliding fee scale program—Parkland Financial Assistance—providing access to primary and subspecialty medical care (including CRC screening, follow-up diagnostic testing, and treatment evaluation) for uninsured Dallas County residents in Texas.
The study was approved by University of Texas Southwestern’s institutional review board (Supplement 1). For this pragmatic trial, the University of Texas Southwestern institutional review board deemed a waiver of consent to be ethical and appropriate because (1) the study was minimal risk because CRC screening is the standard of care and available to all persons through usual care, (2) a waiver of consent would not adversely affect the rights and welfare of participants, (3) the large-scale trial would not be feasible if informed consent were required, and (4) conducting a cancer screening trial in only those consenting to participate would have a large volunteer bias threatening its generalizability and validity as a population health strategy. Although prior studies demonstrate the benefits of screening outreach interventions, CRC screening for most persons in the United States (including those at Parkland Health and Hospital System) is delivered through visit-based screening as recommended by clinicians. Participants in the usual care group would not have had outreach withheld if Parkland decided to implement an outreach program as part of usual care during the study period.
As described previously,13 the population included individuals aged 50 to 64 years with Parkland Financial Assistance coverage at randomization and a primary care visit during the year prior to randomization (the trial protocol and statistical analysis plan are available in Supplement 1). Parkland Financial Assistance provides access to CRC screening with co-payments on a sliding scale basis ranging from $0 to $50 depending on level of income. Participants were excluded if they were up to date with screening, including colonoscopy within 10 years, sigmoidoscopy within 5 years, or FIT testing within 1 year as ascertained from Parkland’s comprehensive electronic health records (EHRs) that include all test and procedure results done anywhere in the health system. Exclusion criteria included (1) no contact information; (2) language other than English or Spanish; (3) history of CRC, inflammatory bowel disease, polyps, or colectomy; and (4) incarceration.
Random Assignment
Eligible persons were randomly assigned to either the usual care, FIT outreach, or colonoscopy outreach groups in a 1:2:2 ratio using a computer-generated, simple randomization sequence. Participants were blinded to presence of alternate interventions.
CRC Screening Interventions
Usual care group participants were eligible to receive whatever visit-based screening was recommended and ordered during any in-person outpatient visit. CRC screening test recommendations were at the discretion of clinicians (typically home-based 3-sample FIT [Hemoccult ICT, Beckman Coulter] or colonoscopy) (Supplement 2). Parkland clinics had a visit based CRC screening EHR reminder that flagged individuals who were not up to date with screening and had primary care clinician–level audit and feedback for CRC-screening performance. The study protocol did not include additional clinician education, patient education, or decision aids for persons in the usual care group.
Participants in the FIT outreach group received an invitation letter in both English and Spanish with information regarding CRC risk, a 1-sample home FIT test kit (OC-Auto FIT CHEK; Polymedco) with instructions, and a return envelope with prepaid postage every 12 months during the study period. Using standardized scripts, bilingual research staff telephoned persons who did not return the FIT kit within 2 weeks. Up to 2 attempts to reach each participant by telephone were made by trying home or cell phone numbers recorded in the EHR at different times of the day. Those with abnormal FIT results (cutoff, 100ng/mL) were informed of results by research staff within 1 week and referred for colonoscopy.
Participants in the colonoscopy outreach group received an invitation letter in both English and Spanish with information regarding CRC risk and a phone number to schedule a colonoscopy. Using standardized scripts, bilingual research staff telephoned persons who did not call within 2 weeks. Up to 2 attempts to reach each participant by telephone were made by trying home or cell phone numbers recorded in the EHR at different times of the day. Participants who called were triaged to open-access colonoscopy slots or preprocedure clinical review by research staff based on a structured history form. They were informed colonoscopy may require a co-payment ranging from $0 to $50 depending on income level. If interested, the procedure was scheduled, and research staff mailed a free bowel prep kit (Gatorade [PepsiCo] and polyethylene glycol 3350) and preprocedure instructions. Research staff called participants 10 days and 2 days prior to colonoscopy to review prep instructions and address preprocedural questions. Participants who did not complete colonoscopy on the basis of EHR data (eTable in Supplement 2) in the Parkland health system received repeat invitation letters each year.
Participants in the outreach groups were eligible for visit-based screening through usual care. Although clinicians may have been aware of the pragmatic trial, they had no knowledge of group assignment unless the participant shared the letter with his or her clinician.
Outcome Measures
The primary outcome—screening process completion within 3 years—was defined as (1) colonoscopy completed with no cancer detected; (2) cancer detected by colonoscopy and treatment evaluation completed within 2 or fewer months; (3) a normal FIT result repeated annually for 3 years (ie, every 12 months anchored on randomization date); (4) an abnormal FIT result with colonoscopy completed within 6 or fewer months with no cancer detected; or (5) an abnormal FIT result with cancer detected by colonoscopy and treatment evaluation completed within 2 or fewer months.12 All participants were randomized in year 1 (March 2013–January 2014) and followed up through July 2016 for screening receipt. The original study outcome was screening process completion within 40 months. However, the institutional review board protocol was amended in June 2016 to terminate follow-up in July 2016 because Parkland Hospital and its central laboratory were scheduled to close down and move into a new facility. This change would have prevented protocol-specified timely processing and reporting of mailed FIT kits. To maintain the fidelity of the intervention and subsequent comparisons of comparative effectiveness, follow-up time was reduced for outcome ascertainment (Supplement 2). After the modification, the primary study outcome time frame was modified to within 3 years, and actual follow-up time for participants ranged from 30 to 40 months depending on the initial screening invite wave.
Colonoscopies with poor prep or without cecal intubation are insufficient and thus were not considered toward screening process completion, nor were FITs that could not be processed (eg, too old or an inadequate specimen). Tests completed as a result of outreach as well as those completed through usual care for any indication were included. Sociodemographics, clinical characteristics, and study outcomes were ascertained from Parkland’s EHRs, as was screening participation (ie, both test orders and results). Race/ethnicity in the EHR was based on self-report at the time of initial patient registration and categorized as non-Hispanic white, black, Hispanic white, Asian, or other. This data was included to help describe the study population. Race/ethnicity was included because of the higher CRC incidence and lower screening rates in racial/ethnic minorities.
Secondary outcomes, defined a priori, included (1) proportions of participants with any adenomas and advanced neoplasia and (2) screening harms, including perforation or post-colonoscopy bleeding. Polyp histology was determined from pathology records, with interpretations performed by gastrointestinal- trained pathologists through routine care. Advanced neoplasia was defined as adenomas larger than 1 cm, sessile serrated adenomas, adenomas containing high-grade dysplasia or villous histology, or invasive CRC. Presence of adenomas and advanced neoplasia were determined using EHR data; CRC cases were confirmed to not be missed by EHR data by checking state registry data. Cost-effectiveness of each strategy for promoting screening process completion was an a priori outcome but is not included in this article.
Three post hoc secondary analyses were performed using more-liberal definitions of screening process completion. First, an analysis was performed comparing screening process completion among participants screened through outreach efforts. Second, screening completion was defined using biennial FIT in years 1 and 3 or years 1 and 2, as recommended in Europe,14 for those with negative results. Participants with abnormal FIT results were still required to undergo colonoscopy within 6 months. Third, comparison of receipt of any screening (≥1 FIT or colonoscopy) over the 3-year period was performed. A post hoc descriptive analysis of process failures was performed at each step in the screening process.
Statistical Analysis
The Pearson χ2 test was used to compare screening process completion, screening process failures, and screening outcomes (eg, number of adenomas) between groups. Sample size calculations were determined a priori to compare screening process completion across groups. As described in Supplement 2, assumptions for proportions completing each step of the screening process were based on available medical literature (eReferences 1–12 in Supplement 2). Under these assumptions, the predicted screening process completion rates were 16.6% and 13.5% for the outreach groups, yielding a difference of 3.1%. Although no prior studies compared FIT and colonoscopy outreach with the outcome of screening process completion, prior studies of successful CRC screening interventions report differences in screening rates exceeding 3.1%.15 With 2400 persons assigned to the FIT outreach group and 2400 to the colonoscopy outreach group, an estimated 80% power was needed to detect between-group differences of at least 3.1% in screening process completion, assuming screening process completions of 13.5% for colonoscopy outreach and 16.6% for FIT outreach and a prespecified 2-sided α of .05. After Bonferroni correction to adjust for comparisons between the 3 groups, an estimated 67% power was needed to detect a difference in screening process completion proportions between the 2 intervention groups and more than 95% power to detect differences between both intervention groups and the usual care group at a 2-sided significance level of .017 (Supplement 2). Participants without any subsequent visits at Parkland Health and Hospital System were labeled as lost to follow-up but retained in all analyses, consistent with the plan for an intention-to-screen analysis. Missing data were rare and reported as unknown. An intention-to-screen principle guided analyses, which were conducted with SAS (SAS Institute), version 9.4.
Results
Study Population
Of 5999 individuals eligible for inclusion (median age, 56 years; women, 62%), 2400 were assigned to the FIT outreach group, 2400 to the colonoscopy outreach group, and 1199 to the usual care group (Figure 1 and Table 1). Participants were Hispanic (49%), black (24%), white (22%), and had unknown race/ethnicity (0.6%). Of the randomized participants, 39% preferred the Spanish language, 7.0% had a Charlson comorbidity score higher than 2, and all had 1 or more primary care visit the year prior to randomization (nearly one-third had >3 visits). Less than 2% received gastrointestinal subspecialty care the year before or after random assignment.
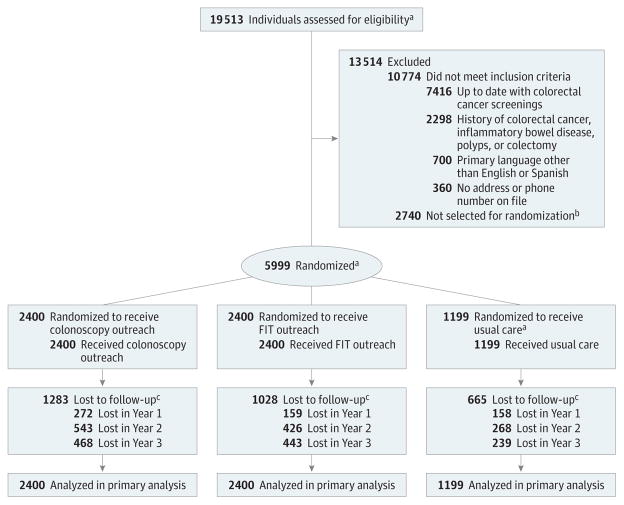
Figure 1. Flow of Participants Through the Study.
FIT indicates fecal immunochemical test.
aOverall 19 514 individuals were assessed for eligibility and 6000 were selected for randomization. One individual was randomized twice to the usual care group due to multiple medical record numbers; therefore, the individual was excluded from the Figure.
bPer a priori power calculations, the target sample size was 6000 individuals. A random sample of 6000 individuals was taken from the eligible population (n = 8740); therefore, 2740 individuals were not selected for randomization.
cLost to follow-up was defined as a lack of subsequent visits at Parkland Health and Hospital System (Dallas, Texas).
Table 1.
Characteristics of Adult Participants Receiving Care at an Urban Safety-Net Hospital Who Were Not Up to Date With Colorectal Cancer Screenings by Study Group
| FIT Outreach, No. of Participants (%)(n = 2400) | Colonoscopy Outreach, No. of Participants (%)(n = 2400) | Usual Care, No. of Participants (%)(n = 1199) | |
|---|---|---|---|
| Age, median (IQR), y | 56 (53–60) | 55 (52–60) | 55 (52–59) |
| Women | 1474 (61.4) | 1494 (62.3) | 744 (62.1) |
| Race/ethnicity | |||
| Non-Hispanic white | 514 (21.4) | 540 (22.5) | 261 (21.8) |
| Hispanic | 1162 (48.4) | 1184 (49.3) | 585 (48.8) |
| Black | 594 (24.8) | 558 (23.3) | 272 (22.7) |
| Other or unknown | 130 (5.4) | 118 (4.9) | 81 (6.8) |
| Language | |||
| English | 1478 (61.6) | 1451 (60.5) | 728 (60.7) |
| Spanish | 922 (38.4) | 949 (39.5) | 471 (39.3) |
| Charlson Comorbidity Indexa | |||
| 0 | 1155 (48.1) | 1151 (48.0) | 553 (46.1) |
| 1 | 887 (37.0) | 901 (37.5) | 456 (38.0) |
| 2 | 192 (8.0) | 181 (7.5) | 103 (8.6) |
| ≥3 | 166 (6.9) | 167 (7.0) | 87 (7.3) |
| Annual primary care physician visits, median (IQR), No. | |||
| Year prior to randomization | 2 (1–3) | 2 (1–3) | 2 (1–3) |
| Year after randomization | 1 (0–3) | 2 (0–3) | 1 (0–3) |
| Receiving ≥1 gastrointestinal subspecialty visit, median (IQR) | |||
| Year prior to randomization | 20 (0.8) | 26 (1.1) | 13 (1.1) |
| Year after randomization | 35 (1.5) | 35 (1.5) | 18 (1.5) |
Abbreviation: FIT, fecal immunochemical test; IQR, interquartile range.
Charlson Comorbidity Index was derived using International Classification of Diseases, Ninth Revision, codes from the Parkland Health and Hospital System (Dallas, Texas) electronic health record data during the 12 mo prior to cohort entry.
Primary Outcome
Screening process completion was achieved in 38.4% (95% CI, 36.5% to 40.4%) of participants in the colonoscopy outreach group, 28.0% (95% CI, 26.2% to 29.8%) in the FIT outreach group, and 10.7% (95% CI, 9.1% to 12.6%) in the usual care group (Table 2). Compared with the usual care group, screening process completion was 27.7% (95% CI, 25.1% to 30.4%) higher in the colonoscopy outreach group and 17.3% (95% CI, 14.8% to 19.8%) higher in the FIT outreach group and 10.4% (95% CI, 7.8% to 13.1%) higher for the colonoscopy outreach group compared with the FIT outreach group (P < .001 for all comparisons).
Table 2.
Proportion of Adult Participants Receiving Care at an Urban Safety-Net Hospital Who Were Not Up to Date With Colorectal Cancer Screenings Who Completed the Colorectal Cancer Screening Processa Within 3 Years by Study Group
| FIT Outreach, % (95% CI) (n = 2400) | Colonoscopy Outreach, % (95% CI) (n = 2400) | Usual Care, % (95% CI) (n = 1199) | |
|---|---|---|---|
| Completed screening process, No. | 671 | 922 | 128 |
| Screening process completion rates | 28.0 (26.2–29.8) | 38.4 (36.5–40.4) | 10.7 (9.1–12.6) |
| Differences between groups in screening process completion rates | |||
| vs usual care | 17.3 (14.8–19.8) | 27.7 (25.1–30.4) | Reference |
| vs FIT outreach | Reference | 10.4 (7.8–13.1) | 17.3 (14.8–19.8) |
Abbreviation: FIT, fecal immunochemical test.
Screening process completion was a composite outcome defined as 1 of the following: (1) colonoscopy completed with no cancer detected, (2) cancer detected by colonoscopy and treatment evaluation completed within 2 mo, (3) negative FIT result repeated annually for 3 y, (4) positive FIT result and colonoscopy completed within 6 mo with no cancer detected, or (5) positive FIT result, cancer detected by colonoscopy, and treatment evaluation completed within 2 mo.
Secondary Outcomes
Adenoma and advanced neoplasia detection rates differed by study group (Table 3). Adenomas were detected in 344 participants (14.3%)in the colonoscopy outreach group, 128 (5.3%) in the FIT outreach group, and 48 (4.0%) in the usual care group. Advanced neoplasia was detected in 105 participants (4.4%)in the colonoscopy outreach group, 49 (2.0%)in the FIT outreach group, and 16 (1.3%) in the usual care group. Compared with the usual care group, detection rates were 10.3% (95%CI, 9.5% to 12.1%) higher for adenoma and 3.1%(95%CI, 2.0% to 4.1%) higher for advanced neoplasia in the colonoscopy outreach group(P < .001 for both) and 1.3%(95%CI, −0.1% to 2.8%) higher for adenoma and 0.7%(95%CI, −0.2% to 1.6%) higher for advanced neoplasia in the FIT outreach group (P = .08 and P = .13, respectively). The differences between outreach groups were 9.0%(95%CI, 7.3% to 10.7%) for adenoma and 2.4% (95% CI, 1.3% to 3.3%) for advanced neoplasia (P < .001 for both). Among participants with any screening, the colonoscopy outreach group yielded 19.2%(95%CI, 16.4% to 22.0%) higher adenoma detection and 5.0% (95%CI, 3.3% to 6.8%) higher advanced neoplasia detection than the FIT outreach group. Among those who completed the screening process, adenoma and advanced neoplasia detection rates were 17.8% (95% CI, 13.5% to 22.1%) higher for adenoma and 3.7% (95%CI, 0.8% to 6.5%) higher for advanced neoplasia for the colonoscopy outreach group vs the FIT outreach group. There were not any screening-related harms in any group.
Table 3.
Colorectal Cancer Screening Outcomes Among Adult Participants Receiving Care at an Urban Safety-Net Hospital Who Were Not Up to Date With Colorectal Cancer Screenings by Study Group
| FIT Outreach, No. of Participants (%)(n = 2400) | Colonoscopy Outreach, No. of Participants (%)(n = 2400) | Usual Care, No. of Participants (%)(n = 1199) | |
|---|---|---|---|
| Any polyp | 168 (7.0) | 504 (21.0) | 64 (5.3) |
| Any adenoma | 128 (5.3) | 344 (14.3) | 48 (4.0) |
| Advanced neoplasiaa | 49 (2.0) | 105 (4.4) | 16 (1.3) |
| Advanced adenomaa | 42 (1.7) | 95 (4.0) | 13 (1.1) |
| Colorectal cancer | 7 (0.3) | 10 (0.4) | 3 (0.2) |
Abbreviation: FIT, fecal immunochemical test.
Advanced neoplasia was defined as advanced adenoma (adenoma >1 cm or with high-grade dysplasia or villous histology) or colorectal cancer.
In a post hoc analysis only including participants screened through outreach efforts, screening process completion was 15.4% (95% CI, 12.8% to 18.0%) higher for the colonoscopy outreach group than the FIT outreach group (33.7% for the colonoscopy outreach group vs 18.3% for the FIT outreach group, P < .001). Of those who completed the screening process, only 1.0% (n = 9) in the colonoscopy outreach group completed an annual usual care FIT, whereas 33.1% (n = 222) in the FIT outreach group instead completed colonoscopy via usual care.
In post hoc analyses with less-stringent definitions for screening process completion, the colonoscopy outreach group had 0.5% (95% CI, −2.2% to 3.4%) lower screening process completion than the FIT outreach group if requiring biennial FIT (41.0% for the colonoscopy outreach group vs 41.5% the FIT outreach group, P = .68). Any screening receipt over the study period was intermediate (51.7% [95% CI, 49.7% to 53.7%]) in the colonoscopy outreach group; highest (65.0% [95% CI, 63.0% to 66.8%]) in the FIT outreach group and lowest (39.0% [95% CI, 36.2% to 41.7%]) in the usual care group.
Screening Process Failures
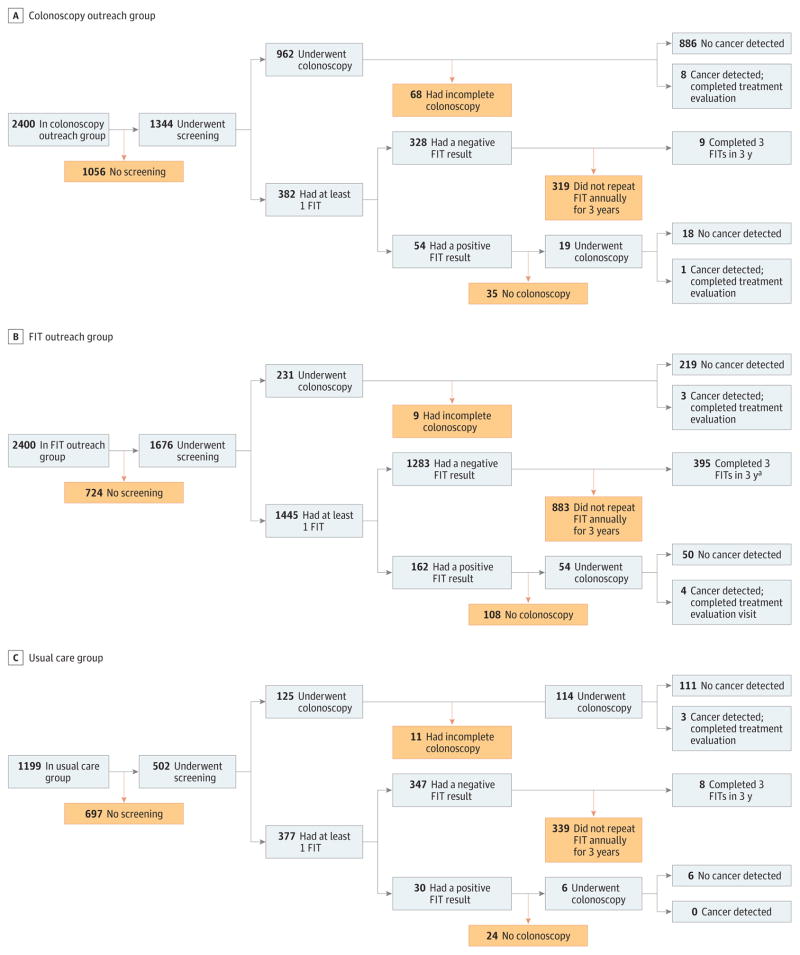
There were screening process failures at multiple steps in the screening process in each group (Figure 2). In the colonoscopy outreach group, nearly half of the participants (n = 1056; 44.0%) did not initiate screening, 27 (1.1%) had incomplete colonoscopy without cecal intubation, 23 (1.0%) had inadequate prep quality, and 18 (0.8%) had both incomplete colonoscopy and inadequate prep quality. Among participants in the colonoscopy outreach group who opted for FIT via usual care, 319 only underwent intermittent FIT (with 250 undergoing 1 FIT and 69 undergoing FIT in only 2 of 3 years). Among 54 participants with an abnormal FIT result, 35 (64.8%) did not have a timely follow-up colonoscopy (22 underwent colonoscopy after 6 months, 12 did not undergo colonoscopy, and 1 had an inadequate colonoscopy due to a lack of cecal intubation). All participants in the colonoscopy outreach group who were found to have CRC (n = 10) had a timely treatment evaluation.
Figure 2. Flow of Incomplete Colorectal Cancer Screening Processes by Study Group.
FIT indicates fecal immunochemical test. Yellow boxes indicate screening process failures.
aParticipants who completed 3 FITs in 3 years did so by outreach only (60%; n = 237), combination of outreach and usual care (39.2%; n = 155), and usual care only (0.8%; n = 3).
Among participants assigned to the FIT outreach group, 724 (30.2%) did not initiate screening. More than one-third (n = 888; 37.0%) of participants only underwent intermittent FIT (522 had 1 FIT and 366 had FIT in only 2 of 3 years). Among 162 participants with an abnormal FIT result, 108 (66.7%) did not have a timely follow-up colonoscopy (17 underwent colonoscopy after 6 months, 83 did not undergo colonoscopy, and 8 had inadequate colonoscopies due to a lack of cecal intubation or inadequate prep quality). Of participants in the FIT outreach group who opted for colonoscopy via usual care, 2 were incomplete without cecal intubation and 7 had inadequate prep quality. All participants in the FIT outreach group who were found to have CRC (n = 7) had a timely treatment evaluation.
Among participants in the usual care group, 697 (58.1%) did not initiate screening, 4 (0.3%) underwent colonoscopy without cecal intubation, and 7 (0.6%) had inadequate prep quality. There were 339 (28.3%) participants who only underwent intermittent FIT screening, and among the 30 participants with an abnormal FIT result, 24 (80%) did not have a timely follow-up colonoscopy (10 underwent colonoscopy after 6 months and 14 did not undergo colonoscopy). All 3 usual care participants (0.3%) with CRC had a timely treatment evaluation.
Discussion
In this pragmatic randomized clinical trial among adults aged 50 to 64 years who were receiving primary care in an urban safety-net health system, both mailed outreach interventions encouraging FIT and encouraging colonoscopy increased the proportion of individuals who completed the CRC screening process within 3 years compared with usual care. Colonoscopy outreach was more effective than FIT outreach for screening process completion, from screening initiation to repeat screening and diagnostic evaluation. However, screening process completion for both outreach groups remained below 40%, highlighting the potential for further improvement. Higher screening process completion among participants in the colonoscopy outreach group translated to higher adenoma and advanced neoplasia detection, although there was no difference in cancer detection.
Although the primary outcome of screening process completion is a more rigorous and clinically important outcome than 1-time screening, it remains an imperfect surrogate for reduced mortality—the ultimate goal of cancer screening. There was a higher proportion of adenomas and advanced neoplasia in the colonoscopy outreach group; however, the trial was not powered to evaluate differences in early CRC detection. The CONFIRM16 and COLONPREV17 trials are ongoing multicenter studies comparing colonoscopy and FIT for early CRC detection and mortality, but they are years away from reporting. In the absence of data on long-term outcomes for FIT-based vs colonoscopy-based screening strategies, these results suggest colonoscopy outreach may be particularly effective for promoting screening process completion in health systems with sufficient endoscopic capacity.
Although studies suggest FIT invitations are more effective than colonoscopy outreach for 1-time screening,3,18 participants in this study who were offered colonoscopy outreach had higher screening process completion and adenoma detection when followed for a longer duration. FIT has lower barriers to 1-time participation19,20 but requires annual screening and diagnostic evaluation of abnormal results. In contrast, colonoscopy is both a screening and diagnostic test, so a single examination can satisfy screening process completion for up to 10 years. Although colonoscopy outreach was the most-effective outreach strategy, this may not be scalable to larger at-risk populations within health systems due to finite endoscopic capacity,18,21 greater costs, and potential procedure related complications.22,23 Although the study’s follow-up period was longer than most studies, it was not sufficient to determine whether participants who had adenomas removed underwent appropriate follow-up surveillance examinations. Similarly, FIT outreach may not be scalable in all health systems due to costs.
Although the outreach strategies increased screening process completion compared with usual care, screening completion remained below 40% in both outreach groups. The types of screening process failures at each step differed by test. Although nearly half of the participants in the colonoscopy outreach group did not initiate screening, there were minimal downstream process failures among participants who did. Fewer participants undergoing colonoscopy may complete the screening process when considering longer periods because 3-year and 5-year surveillance colonoscopies would be required for those with higher-risk polyps. Prior studies show under use of surveillance colonoscopy in up to 50% of eligible patients.24–26 In contrast, nearly 70% of participants in the FIT outreach group initiated screening but over one-third did not undergo repeat screening despite free annual mailed kits. Two-thirds of participants in the FIT outreach group also did not undergo diagnostic evaluation after abnormal FIT results. These data are consistent with prior studies demonstrating prevalent screening process failures, with 25% to 70% of patients who complete the initial FIT not undergoing repeat testing and 20% to 50% of those with an abnormal FIT result not receiving timely diagnostic evaluation.4–6,27–29 However, follow-up of abnormal FITs in this study was at the lower end of the spectrum, highlighting the need to address these screening process failures to improve FIT effectiveness.
These data can help clinicians weigh the pros and cons of different CRC screening strategies in their patient population and practice environment. It is important to consider patients’ barriers to screening initiation when recommending colonoscopy and the need for annual screening or diagnostic colonoscopy when recommending FIT. From the health system perspective, data on screening process failures can help identify intervention targets to improve screening effectiveness. Initiating colonoscopy screening may be improved by patient-level (transportation assistance) or system-level (reducing co-payment costs) interventions.30–32 Studies have demonstrated patient-level (education) and system-level (automated reminders) interventions can improve repeat FIT completion.10,33 Moreover, these data can inform simulation studies comparing colonoscopy and FIT as system-level screening strategies. A decision analysis conducted by the USPSTF concluded annual FIT provides similar life-years gained as colonoscopy every 10 years, but this assumed 100% adherence with screening and diagnostic evaluation for strategies, so more-nuanced modeling studies are needed.34
Although both outreach strategies significantly increased screening completion compared with usual care, screening process completion remained below 40% in both groups. Thirty-three percent of participants assigned to the FIT outreach group who completed the screening process did so via usual care colonoscopy, which suggests providing a choice of screening modalities and shared decision making may be an effective strategy.35–37 Inadomi and colleagues38 found that providing patients a choice of screening modality increased screening participation, although not statistically significant, compared with only offering a fecal occult blood test. However, participation in the study required consent, potentially selecting for those interested in CRC screening and introducing volunteer bias, so it is possible providing choice would have greater effect in a pragmatic trial setting.
This study has several strengths including its large sample size, racially and socioeconomically diverse patient population, pragmatic study design avoiding volunteer bias, and innovation comparing colonoscopy vs FIT outreach to promote screening process completion over several years.
Limitations
This study has several limitations. First, because this was a pragmatic trial, mailed invitations were simple, 1-page letters and not in-depth decision aids. Second, the trial included reserved colonoscopy appointment slots, which may not be feasible if scaled to larger at-risk populations. However, the number of slots for colonoscopies were designated to mirror waiting times for usual care participants (median time from order to colonoscopy appointment, 106 days for colonoscopy outreach patients vs 101 days for usual care patients). Third, participants could have received screening at outside institutions, although this is highly unlikely because nearly all of the participants’ insurance arrangements only covered care within the safety-net health system and these low-income individuals would be very unlikely to pay out of pocket for cancer screening done elsewhere. Fourth, the low absolute rates of screening may be partly explained by participants’ beliefs about CRC screening, moving, not following-up at Parkland, or having contraindications to screening, which may be underrecognized given the pragmatic design of the trial. Fifth, differences in adenoma and advanced neoplasia detection were partly driven by low proportions of diagnostic colonoscopy after an abnormal FIT result but reflect challenges associated with screening with noninvasive tests such as FIT. Sixth, colonoscopy indication could not be ascertained so some colonoscopies in each group may have been diagnostic. In addition, the study was not powered to detect differences in CRC detection or mortality.
Conclusions
Among persons aged 50 to 64 years receiving primary care at a safety-net institution, mailed outreach invitations offering FIT or colonoscopy compared with usual care increased the proportion completing CRC screening process within 3 years. The rate of screening process completion was higher with colonoscopy than FIT outreach.
Supplementary Material
Key Points.
Question
Which screening strategy is most effective in promoting colorectal cancer (CRC) screening process completion among individuals who are not up to date with CRC screening?
Findings
In this randomized clinical trial that included 5999 patients who were followed up for 3 years, screening process completion occurred in 28.0% in the mailed fecal immunochemical test (FIT) outreach group, 38.4% in the mailed colonoscopy outreach group, and 10.7% in the usual care group; the result for each intervention was significantly greater than for usual care.
Meaning
Outreach interventions offering FIT or colonoscopy may be more effective than usual care in increasing the proportion of persons who complete the CRC screening process.
Acknowledgments
Funding/Support: This study was conducted as part of the National Cancer Institute (NCI)–funded consortium Population-Based Research Optimizing Screening through Personalized Regiments (PROSPR) with support from grants U54CA163308-01 and P30 CA142543 from the National Institutes of Health (NIH) and NCI and grant NIH UL1TR001105 from the NIH. This work was also supported in part by grant R24 HS022418 the Agency for Healthcare Research and Quality (AHRQ) Center for Patient-Centered Outcomes Research (Dr Halm). Polymedco provided fecal immunochemical test (FIT) kits and reagents.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or AHRQ.
Author Contributions: Drs Singal and Halm had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Singal, Gupta, Ahn, Santini, Bishop, Halm.
Acquisition, analysis, or interpretation of data: Singal, Gupta, Ahn, Mayorga, Murphy, McCallister, Sanders, Loewen, Halm.
Drafting of the manuscript: Singal, Gupta, Ahn, Murphy, McCallister, Sanders, Loewen, Halm.
Critical revision of the manuscript for important intellectual content: Singal, Gupta, Santini, Mayorga, Murphy, Sanders, Bishop, Halm.
Statistical analysis: Singal, Ahn, Sanders, Loewen.
Obtained funding: Gupta, Bishop, Halm.
Administrative, technical, or material support: Singal, Gupta, Mayorga, McCallister, Sanders, Bishop, Halm.
Supervision: Singal, Santini, McCallister, Bishop, Halm.
Additional Contributions: We thank the Polymedco for providing FIT kits and reagents; the research team from University of Texas Southwestern Medical Center for assistance conducting outreach activities; and our colleagues at Parkland Health and Hospital System and Parkland Center for Clinical Innovation for supporting our PROSPR research initiatives. We also thank Mark Burkart, MA, MS (Parkland Center for Clinical Innovation), for his programming expertise. He did not receive compensation for his contribution.
References
- 1.Bibbins-Domingo K, Grossman DC, Curry SJ, et al. US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;315(23):2564–2575. doi: 10.1001/jama.2016.5989. [DOI] [PubMed] [Google Scholar]
- 2.Gupta S, Halm EA, Rockey DC, et al. Comparative effectiveness of fecal immunochemical test outreach, colonoscopy outreach, and usual care for boosting colorectal cancer screening among the underserved: a randomized clinical trial. JAMA Intern Med. 2013;173(18):1725–1732. doi: 10.1001/jamainternmed.2013.9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White A, Thompson TD, White MC, et al. Cancer screening test use—United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(8):201–206. doi: 10.15585/mmwr.mm6608a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fenton JJ, Elmore JG, Buist DS, Reid RJ, Tancredi DJ, Baldwin LM. Longitudinal adherence with fecal occult blood test screening in community practice. Ann Fam Med. 2010;8(5):397–401. doi: 10.1370/afm.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liss DT, Petit-Homme A, Feinglass J, Buchanan DR, Baker DW. Adherence to repeat fecal occult blood testing in an urban community health center network. J Community Health. 2013;38(5):829–833. doi: 10.1007/s10900-013-9685-x. [DOI] [PubMed] [Google Scholar]
- 6.Chubak J, Garcia MP, Burnett-Hartman AN, et al. PROSPR consortium. Time to colonoscopy after positive fecal blood test in 4 US health care systems. Cancer Epidemiol Biomarkers Prev. 2016;25(2):344–350. doi: 10.1158/1055-9965.EPI-15-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin J, Halm EA, Tiro JA, et al. Reasons for lack of diagnostic colonoscopy after positive result on fecal immunochemical test in a safety-net health system. Am J Med. 2017;130(1):93.e1–93.e7. doi: 10.1016/j.amjmed.2016.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hawley ST, McQueen A, Bartholomew LK, et al. Preferences for colorectal cancer screening tests and screening test use in a large multispecialty primary care practice. Cancer. 2012;118(10):2726–2734. doi: 10.1002/cncr.26551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wheeler SB, Kuo TM, Meyer AM, et al. Multilevel predictors of colorectal cancer testing modality among publicly and privately insured people turning 50. Prev Med Rep. 2016;6:9–16. doi: 10.1016/j.pmedr.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green BB, Anderson ML, Wang CY, et al. Results of nurse navigator follow-up after positive colorectal cancer screening test: a randomized trial. J Am Board Fam Med. 2014;27(6):789–795. doi: 10.3122/jabfm.2014.06.140125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh H, Kadiyala H, Bhagwath G, et al. Using a multifaceted approach to improve the follow-up of positive fecal occult blood test results. Am J Gastroenterol. 2009;104(4):942–952. doi: 10.1038/ajg.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tiro JA, Kamineni A, Levin TR, et al. The colorectal cancer screening process in community settings: a conceptual model for the population-based research optimizing screening through personalized regimens consortium. Cancer Epidemiol Biomarkers Prev. 2014;23(7):1147–1158. doi: 10.1158/1055-9965.EPI-13-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singal AG, Gupta S, Tiro JA, et al. Outreach invitations for FIT and colonoscopy improve colorectal cancer screening rates: a randomized controlled trial in a safety-net health system. Cancer. 2016;122(3):456–463. doi: 10.1002/cncr.29770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stegeman I, van Doorn SC, Mundt MW, et al. Participation, yield, and interval carcinomas in 3 rounds of biennial FIT-based colorectal cancer screening. Cancer Epidemiol. 2015;39(3):388–393. doi: 10.1016/j.canep.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Goebel M, Singal AG, Nodora J, et al. How can we boost colorectal and hepatocellular cancer screening among underserved populations? Curr Gastroenterol Rep. 2015;17(6):22. doi: 10.1007/s11894-015-0445-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quintero E, Castells A, Bujanda L, et al. COLONPREV Study Investigators. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med. 2012;366(8):697–706. doi: 10.1056/NEJMoa1108895. [DOI] [PubMed] [Google Scholar]
- 17.Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370(14):1287–1297. doi: 10.1056/NEJMoa1311194. [DOI] [PubMed] [Google Scholar]
- 18.Seeff LC, Manninen DL, Dong FB, et al. Is there endoscopic capacity to provide colorectal cancer screening to the unscreened population in the United States? Gastroenterology. 2004;127(6):1661–1669. doi: 10.1053/j.gastro.2004.09.052. [DOI] [PubMed] [Google Scholar]
- 19.Xu Y, Levy BT, Daly JM, Bergus GR, Dunkelberg JC. Comparison of patient preferences for fecal immunochemical test or colonoscopy using the analytic hierarchy process. BMC Health Serv Res. 2015;15:175. doi: 10.1186/s12913-015-0841-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med. 2012;172(7):575–582. doi: 10.1001/archinternmed.2012.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilschut JA, Habbema JD, van Leerdam ME, et al. Fecal occult blood testing when colonoscopy capacity is limited. J Natl Cancer Inst. 2011;103(23):1741–1751. doi: 10.1093/jnci/djr385. [DOI] [PubMed] [Google Scholar]
- 22.Denis B, Gendre I, Sauleau EA, Lacroute J, Perrin P. Harms of colonoscopy in a colorectal cancer screening programme with faecal occult blood test: a population-based cohort study. Dig Liver Dis. 2013;45(6):474–480. doi: 10.1016/j.dld.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Heleno B, Thomsen MF, Rodrigues DS, Jorgensen KJ, Brodersen J. Quantification of harms in cancer screening trials: literature review. BMJ. 2013;347:f5334. doi: 10.1136/bmj.f5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schoen RE, Pinsky PF, Weissfeld JL, et al. Utilization of surveillance colonoscopy in community practice. Gastroenterology. 2010;138(1):73–81. doi: 10.1053/j.gastro.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper GS, Kou TD, Barnholtz Sloan JS, Koroukian SM, Schluchter MD. Use of colonoscopy for polyp surveillance in Medicare beneficiaries. Cancer. 2013;119(10):1800–1807. doi: 10.1002/cncr.27990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kahn B, Freeland Z, Gopal P, et al. Predictors of guideline concordance for surveillance colonoscopy recommendations in patients at a safety-net health system. Cancer Causes Control. 2015;26(11):1653–1660. doi: 10.1007/s10552-015-0661-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janda M, Hughes KL, Auster JF, Leggett BA, Newman BM. Repeat participation in colorectal cancer screening utilizing fecal occult blood testing: a community-based project in a rural setting. J Gastroenterol Hepatol. 2010;25(10):1661–1667. doi: 10.1111/j.1440-1746.2010.06405.x. [DOI] [PubMed] [Google Scholar]
- 28.Ferrat E, Le Breton J, Veerabudun K, et al. Colorectal cancer screening: factors associated with colonoscopy after a positive faecal occult blood test. Br J Cancer. 2013;109(6):1437–1444. doi: 10.1038/bjc.2013.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paszat L, Rabeneck L, Kiefer L, Mai V, Ritvo P, Sullivan T. Endoscopic follow-up of positive fecal occult blood testing in the Ontario FOBT Project. Can J Gastroenterol. 2007;21(6):379–382. doi: 10.1155/2007/569689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cavanagh MF, Lane DS, Messina CR, Anderson JC. Clinical case management and navigation for colonoscopy screening in an academic medical center. Cancer. 2013;119(suppl 15):2894–2904. doi: 10.1002/cncr.28156. [DOI] [PubMed] [Google Scholar]
- 31.Wilson FA, Villarreal R, Stimpson JP, Pagán JA. Cost-effectiveness analysis of a colonoscopy screening navigator program designed for Hispanic men. J Cancer Educ. 2015;30(2):260–267. doi: 10.1007/s13187-014-0718-7. [DOI] [PubMed] [Google Scholar]
- 32.Fedewa SA, Goodman M, Flanders WD, et al. Elimination of cost-sharing and receipt of screening for colorectal and breast cancer. Cancer. 2015;121(18):3272–3280. doi: 10.1002/cncr.29494. [DOI] [PubMed] [Google Scholar]
- 33.Baker DW, Brown T, Buchanan DR, et al. Comparative effectiveness of a multifaceted intervention to improve adherence to annual colorectal cancer screening in community health centers: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1235–1241. doi: 10.1001/jamainternmed.2014.2352. [DOI] [PubMed] [Google Scholar]
- 34.US Preventive Services Task Force. [Accessed August 4, 2017];Archived evaluating test strategies for colorectal cancer screening: a decision analysis for the US Preventive Services Task Force. https://www.uspreventiveservicestaskforce.org/Page/SupportingDoc/colorectal-cancer-screening/evaluating-test-strategies-for-colorectal-cancer-screening-a-decision-analysis-for-the-us-preventive-services-task-force.
- 35.Barry MJ, Edgman-Levitan S. Shared decision making—pinnacle of patient-centered care. N Engl J Med. 2012;366(9):780–781. doi: 10.1056/NEJMp1109283. [DOI] [PubMed] [Google Scholar]
- 36.Oshima Lee E, Emanuel EJ. Shared decision making to improve care and reduce costs. N Engl J Med. 2013;368(1):6–8. doi: 10.1056/NEJMp1209500. [DOI] [PubMed] [Google Scholar]
- 37.Joosten EA, DeFuentes-Merillas L, de Weert GH, Sensky T, van der Staak CP, de Jong CA. Systematic review of the effects of shared decision-making on patient satisfaction, treatment adherence and health status. Psychother Psychosom. 2008;77(4):219–226. doi: 10.1159/000126073. [DOI] [PubMed] [Google Scholar]
- 38.Liang PS, Wheat CL, Abhat A, et al. Adherence to competing strategies for colorectal cancer screening over 3 years. Am J Gastroenterol. 2016;111(1):105–114. doi: 10.1038/ajg.2015.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.