Abstract
Chitosan-derived, porous nitrogen-enriched carbonaceous carbon nitride catalyst (PCNx) has been synthesized from marine waste and its use demonstrated in a metal-free heterogeneous selective oxidation of 5-hydroxymethyl-furfural (HMF) to 2,5-furandicarboxylic acid (FDCA) using aerial oxygen under mild reaction conditions.
Introduction
Growing demand for petroleum-derived products due to the waning reserves of fossil resources has prompted researchers to seriously consider the sustainable utilization of high-value chemicals and transportation fuels from readily available biomass resources1–6. 5-hydroxymethylfurfuryl (HMF) is one of the common but important platform chemical derived from carbohydrates and can be further upgraded to a variety of useful entities (Fig. 1) such as 2,5-furandicarboxylic acid (FDCA), 5-hydroxymethyl-2-furancarboxylic acid (HMFCA), 5-formyl-2-furancarboxylic acid (FFCA), maleic anhydride (MA) and 2,5-diformylfuran (DFF)7–11. Among these, FDCA is widely used entity of significant value deployed in the production of bio-based polymers namely polyethylene 2,5-furandicarboxylate (PEF) and fine chemicals12–14. Interestingly, FDCA can be considered a viable substitute for the petroleum-derived terephthalic acid, which is used as an essential molecule in the synthesis of polybutyleneterephthalate (PBT) and polyethylene terephthalate (PET) plastics15,16.
Figure 1.
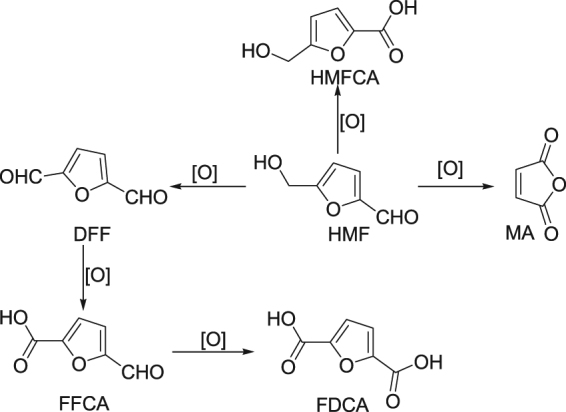
Oxidation products generated from 5-hydroxymethylfurfuryl (HMF).
Earlier, HMF has been fully oxidized to FDCA using toxic oxidants such as nitric acid and potassium permanganate often in stoichiometric quantities generating copious amount of wastes17. Various metal-based homogeneous and heterogeneous catalytic systems have been reported in literature for the direct oxidation of HMF to FDCA18–22. Among homogeneous catalysts, metal-bromide have been reported for the oxidation of HMF23–25. Yet, these catalysts are difficult to separate and frequently lead to the formation of inorganic wastes as by-products that result in environmental contamination. Heterogeneous catalysts such as Pd-, Pt-, and Au-based or bimetallic catalysts including earth abundant metal-based catalysts have also been explored for the synthesis of FDCA under aerobic conditions26–31. However, industrial applications of these metal-based heterogeneous catalysts has not been fruitful in view of higher catalyst cost and the leaching of metal ions into reaction systems making purification more difficult thus culminating in severe environmental pollution. Hence, the search and development of sustainable, cost-effective, metal-free, and efficient heterogeneous catalysts is actively pursued for the aerobic oxidation of HMF to FDCA.
Recently, Wu et al. observed the efficient oxidation of HMF to FDCA with yields of 80% using metal-free N-doped nanoporous graphitic (NNC) catalyst at 80 °C under aerobic condition32; catalyst was synthesized via the pyrolysis of zeolitic imidazole frameworks such as ZIF-8 at 900 °C. However, this method requires catalyst preparation via circuitous route and extended reaction time. In continuous of our work towards the development of sustainable protocol in synthetic transformations33–39, herein, we report a metal-free, efficient method for the aerial oxidation of HMF to FDCA using marine waste originated chitosan-derived porous carbon nitride (PCNx) catalyst as a solid catalyst.
Synthesis and Characterization of Catalyst
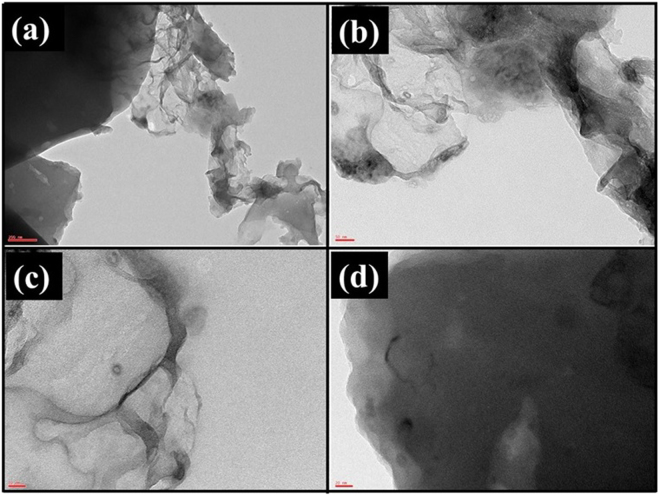
The porous carbon nitride catalyst (PCNx) was synthesized via calcination of chitosan at 300 °C for 4 hours under nitrogen atmosphere. The ensuing PCNx catalyst was characterized using X-ray diffraction (XRD), transmission electron microscope (TEM), and Brunauer–Emmett–Teller (BET) analysis. The XRD patterns of the PCNx catalyst show the characteristic pattern of graphitized carbon (Fig. 2). The graphitic line (002) of the PCNx catalyst was observed at the diffraction peak of 24.97° corresponding to inter-layer spacing of about 0.345 nm which is usually attributed to a high degree of crystallinity of graphitic layers. This XRD pattern also reveals a low content of amorphous carbon and impurities. Additionally, the XRD diffraction peak of 24.97° confirms the presence of glassy carbon known as graphite nitrate supports as described by Afolabi et al.40. TEM analysis shows porous structure of PCNx; wrinkles and bends are easily visible in Fig. 3 which are instigated by various defects. The porous nature of the material was further supported by BET surface area analysis and was found to be 92.83 m2/g.
Figure 2.
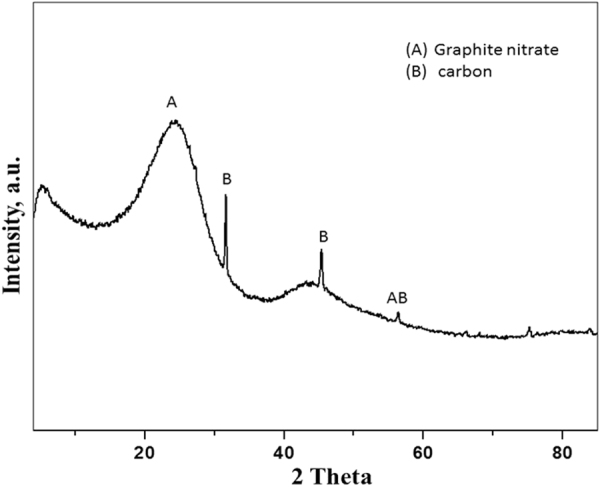
XRD analysis of PCNx catalyst.
Figure 3.
TEM analysis of PCNx catalyst.
Results and Discussion
To study the feasibility for the catalytic aerial oxidation of 5-HMF to FDCA conversion (Fig. 4), different metal-free carbonaceous materials under basic conditions and varying temperature range were evaluated (Table 1, entries 1–22). Various carbon-based catalysts were examined namely graphite, graphene oxide (GO), carbon nanotubes (CNT), and carbon nanofibers (CNF) for the aerial oxidation of 5-HMF to FDCA using water as a solvent and K2CO3 as a base at different temperature (Table 1, entries 1–12); no FDCA production was discerned after 36 hours of reaction (Table 1, entries 1–12). It has been reported that the graphitic nitrogen activates oxygen and plays a central role in the aerobic oxidation of alcohols41. Consequently, we tested N-doped carbon materials that contained graphitic nitrogen as shown in Table 1 (Table 1, entries 13–22). Nitrogen doped graphene gave 5% of FDCA after 36 hours (Table 1, entry 15) whereas graphitic carbon nitride (g-C3N4) afforded only 8% and 15% yields of FDCA at 50 °C and 70 °C, respectively (Table 1, entries 17–18). Nearly quantitative yield of FDCA, however, was obtained when PCNx was used as a catalyst (Table 1, entries 19–22); FDCA yields of 8%, 46%, 83% were observed at 30 °C, 50 °C and 70 °C, respectively (Table 1, entries 19–21). Notably, increasing the reaction temperature to 80 °C, did not give any further improvement in yield (Table 1, entry 22). Furthermore, to understand the effect of base on the reaction, different bases such as NaOH, KOH, Na2CO3 were also evaluated at 70 °C (Table 1, entries 23–25). However, these bases failed to increase the product yield under aerial condition.
Figure 4.
Oxidation of 5-HMF to FDCA.
Table 1.
Screening of catalysts and reaction optimization for FDCA conversiona.
| Entry | Catalyst | Time | Temperature | Yieldb |
|---|---|---|---|---|
| 1 | Graphite | 36 h | 30 °C | — |
| 2 | Graphite | 36 h | 50 °C | — |
| 3 | Graphite | 36 h | 70 °C | — |
| 4 | GO | 36 h | 30 °C | — |
| 5 | GO | 36 h | 50 °C | — |
| 6 | GO | 36 h | 70 °C | — |
| 7 | CNT | 36 h | 30 °C | — |
| 8 | CNT | 36 h | 50 °C | — |
| 9 | CNT | 36 h | 70 °C | — |
| 10 | CNF | 36 h | 30 °C | — |
| 11 | CNF | 36 h | 50 °C | — |
| 12 | CNF | 36 h | 70 °C | — |
| 13 | N-doped graphene | 36 h | 30 °C | — |
| 14 | N-doped graphene | 36 h | 50 °C | traces |
| 15 | N-doped graphene | 36 h | 70 °C | 5% |
| 16 | g-C3N4 | 36 h | 30 °C | — |
| 17 | g-C3N4 | 36 h | 50 °C | 8% |
| 18 | g-C3N4 | 36 h | 70 °C | 15% |
| 19 | PCNx | 36 h | 30 °C | 8% |
| 20 | PCNx | 36 h | 50 °C | 46% |
| 21 | PCNx | 36 h | 70 °C | 83% |
| 22 | PCNx | 48 h | 80 °C | 83% |
| 23c | PCNx | 36 h | 70 °C | 79% |
| 24d | PCNx | 36 h | 70 °C | 80% |
| 25e | PCNx | 36 h | 70 °C | 77% |
a) Reaction condition: 5-HMF (1.0 mmol), water (10.0 ml), PCNx (20 mg), K2CO3 (1.0 mmol); b) Isolated yield; c) NaOH (1.0 mmol); d) KOH (1.0 mmol); e) Na2CO3 (1.0 mmol).
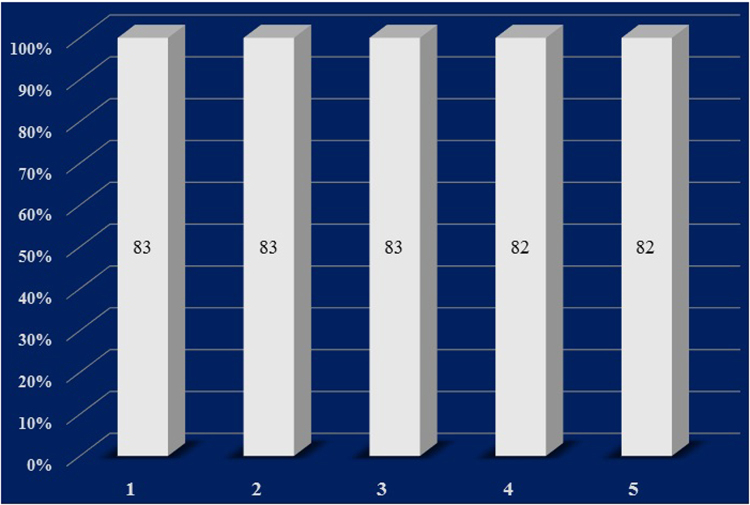
Recycling of chitosan-derived porous CNX catalyst for the aerial oxidation of 5-HMF to FDCA
A set of experiments were performed using 5-HMF in water. After the completion of each reaction, the CNX catalyst was recovered using membrane (0.47 μm pore size) filter, washed with water and reused for the oxidation of a fresh batch of 5-HMF. The CNX catalyst could be recycled and reused up to five times without any loss in its activity (Fig. 5). The XRD analysis of the CNX catalyst before and after the reaction confirms that there is no significant change in the pattern/morphology of the catalyst, which signifies high stability of CNX during the course of the reaction (Supporting Information).
Figure 5.
Recycling of chitosan derived porous CNX catalyst.
Conclusion
We have developed an efficient, sustainable, cost-effective and metal-free protocol for the aerial oxidation of 5-HMF to FDCA using marine waste originated chitosan-derived porous CNx catalyst under mild reaction conditions. This highly active PCNx has been synthesized via calcination of the chitosan at 300 °C under nitrogen atmosphere. The graphitic nitrogen in PCNx activates the oxygen and plays a key role in the aerobic oxidation of alcohols; the oxidation of 5-HMF to FDCA is accomplished in high yield (83%) under ambient air pressure at 70 °C. The PCNx catalyst shows very good recyclability and no significant loss of activity has been observed up to the fifth run.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency. Any mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Electronic supplementary material
Acknowledgements
SV was supported by the Postgraduate Research Program at the National Risk Management Research Laboratory administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the U.S. Environmental Protection Agency.
Author Contributions
S.V. designed and conducted experiments and performed data analysis. S.V., M.N.N. and R.S.V. wrote the manuscript. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-14016-5.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sen SM, et al. A sulfuric acid management strategy for the production of liquid hydrocarbon fuels via catalytic conversion of biomass-derived levulinic acid. Energy Environ. Sci. 2012;5:9690–9697. doi: 10.1039/c2ee22526c. [DOI] [Google Scholar]
- 2.Wang Y, Liu B, Huang K, Zhang Z. Aerobic oxidation of biomass-derived 5-(Hydroxymethyl)furfural into 2,5-diformylfuran catalyzed by the trimetallic mixed oxide (Co–Ce–Ru) Ind. Eng. Chem. Res. 2014;53:1313–1319. doi: 10.1021/ie4034363. [DOI] [Google Scholar]
- 3.Bozell JJ, Petersen GR. Technology development for the production of bio-based products from bio-refinery carbohydrates-the US Department of Energy’s “Top 10” revisited. Green Chem. 2010;12:539–554. doi: 10.1039/b922014c. [DOI] [Google Scholar]
- 4.Chheda JN, Huber GW, Dumesic JA. Liquid-phase catalytic processing of biomass-derived oxygenated hydrocarbons to fuels and chemicals. Angew. Chem., Int. Ed. 2007;46:7164–7183. doi: 10.1002/anie.200604274. [DOI] [PubMed] [Google Scholar]
- 5.An D, et al. Selective conversion of cellobiose and cellulose into gluconic acid in water in the presence of oxygen, catalyzed by polyoxometalate-supported gold nanoparticles. Chem. Eur. J. 2012;18:2938–2947. doi: 10.1002/chem.201103262. [DOI] [PubMed] [Google Scholar]
- 6.Serrano-Ruiz JC, Luque R, Sepúlveda-Escribano A. Transformations of biomass-derived platform molecules: from high added-value chemicals to fuels via aqueous-phase processing. Chem. Soc. Rev. 2011;40:5266–5281. doi: 10.1039/c1cs15131b. [DOI] [PubMed] [Google Scholar]
- 7.Putten RV, et al. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem. Rev. 2013;113:1499–1597. doi: 10.1021/cr300182k. [DOI] [PubMed] [Google Scholar]
- 8.Choudhary V, et al. Insights into the interplay of Lewis and Brønsted acid catalysts in glucose and fructose conversion to 5-(Hydroxymethyl)furfural and levulinic acid in aqueous media. J. Am. Chem. Soc. 2013;135:3997–4006. doi: 10.1021/ja3122763. [DOI] [PubMed] [Google Scholar]
- 9.Corma A, Iborra S, Velty A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007;107:2411–2502. doi: 10.1021/cr050989d. [DOI] [PubMed] [Google Scholar]
- 10.Deng L, et al. Catalytic conversion of biomass-derived carbohydrates into gamma-valerolactone without using an external H2 supply. Angew. Chem., Int. Ed. 2009;48:6529–6532. doi: 10.1002/anie.200902281. [DOI] [PubMed] [Google Scholar]
- 11.Yi G, Teong SP, Li X, Zhang Y. Purification of biomass-derived 5-hydroxymethylfurfural and its catalytic conversion to 2,5-furandicarboxylic acid. ChemSusChem. 2014;7:2131–2135. doi: 10.1002/cssc.201402105. [DOI] [PubMed] [Google Scholar]
- 12.Eerhart AJJE, et al. Fuels and plastics from lignocellulosic biomass via the furan pathway; a technical analysis. RSC Adv. 2014;4:3536–3549. doi: 10.1039/C3RA43512A. [DOI] [Google Scholar]
- 13.Ma J, et al. The copolymerization reactivity of diols with 2,5-furandicarboxylic acid for furan-based copolyester materials. J. Mater. Chem. 2012;22:3457–3481. doi: 10.1039/c2jm15457a. [DOI] [Google Scholar]
- 14.Eerhart AJJE, Faaij APC, Patel MK. Replacing fossil based PET with biobased PEF; process analysis, energy and GHG balance. Energy Environ. Sci. 2012;5:6407–6422. doi: 10.1039/c2ee02480b. [DOI] [Google Scholar]
- 15.Dutta S, De S, Saha B. A brief summary of the synthesis of polyester building-block chemicals and biofuels from 5-hydroxymethylfurfural. ChemPlusChem. 2012;77:259–272. doi: 10.1002/cplu.201100035. [DOI] [Google Scholar]
- 16.Zhu Y, Romain C, Williams CK. Sustainable polymers from renewable resources. Nature. 2016;540:354–362. doi: 10.1038/nature21001. [DOI] [PubMed] [Google Scholar]
- 17.Toshinari, M., Hirokazu, K., Takenobu, K. & Hirohide, M. Method for producing furan-2,5-dicarboxylic acid. U.S. Patent 7,411,078 (2008).
- 18.Ma J, Du Z, Xu J, Chu Q, Pang Y. Efficient aerobic oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran, and synthesis of a fluorescent material. ChemSusChem. 2011;4:51–54. doi: 10.1002/cssc.201000273. [DOI] [PubMed] [Google Scholar]
- 19.Casanova O, Iborra S, Corma A. Biomass into chemicals: aerobic oxidation of 5-hydroxymethyl-2-furfural into 2,5-furandicarboxylic acid with gold nanoparticle catalysts. ChemSusChem. 2009;2:1138–1144. doi: 10.1002/cssc.200900137. [DOI] [PubMed] [Google Scholar]
- 20.Carlini C, et al. Selective oxidation of 5-hydroxymethyl-2-furaldehyde to furan-2,5-dicarboxaldehyde by catalytic systems based on vanadyl phosphate. Appl. Catal., A. 2005;289:197–204. doi: 10.1016/j.apcata.2005.05.006. [DOI] [Google Scholar]
- 21.Davis SE, et al. Oxidation of 5-hydroxymethylfurfural over supported Pt, Pd and Au catalysts. Catal. Today. 2011;160:55–60. doi: 10.1016/j.cattod.2010.06.004. [DOI] [Google Scholar]
- 22.Gorbanev YY, et al. Gold-catalyzed aerobic oxidation of 5-hydroxymethylfurfural in water at ambient temperature. ChemSusChem. 2009;2:672–675. doi: 10.1002/cssc.200900059. [DOI] [PubMed] [Google Scholar]
- 23.Partenheimer W, Grushin VV. Synthesis of 2,5-diformylfuran and furan-2,5-dicarboxylic acid by catalytic air-oxidation of 5-hydroxymethylfurfural. unexpectedly selective aerobic oxidation of benzyl alcohol to benzaldehyde with metal = bromide Catalysts. Adv. Synth. Catal. 2001;343:102–111. doi: 10.1002/1615-4169(20010129)343:1<102::AID-ADSC102>3.0.CO;2-Q. [DOI] [Google Scholar]
- 24.Saha B, Dutta S, Abu-Omar MM. Aerobic oxidation of 5-hydroxylmethylfurfural with homogeneous and nanoparticulate catalysts. Catal. Sci. Technol. 2012;2:79–81. doi: 10.1039/C1CY00321F. [DOI] [Google Scholar]
- 25.Zuo X, Venkitasubramanian P, Busch DH, Subramaniam B. Optimization of Co/Mn/Br-catalyzed oxidation of 5-hydroxymethylfurfural to enhance 2, 5-furandicarboxylic acid yield and minimize substrate burning. ACS Sustainable Chem. Eng. 2016;4:3659–3668. doi: 10.1021/acssuschemeng.6b00174. [DOI] [Google Scholar]
- 26.Mittal N, et al. One-pot synthesis of 2, 5-diformylfuran from fructose using a magnetic bi-functional catalyst. RSC Adv. 2016;6:25678–25688. doi: 10.1039/C6RA01549B. [DOI] [Google Scholar]
- 27.Sádaba I, et al. Catalytic performance of zeolite-supported vanadia in the aerobic oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran. ChemCatChem. 2013;5:284–293. doi: 10.1002/cctc.201200482. [DOI] [Google Scholar]
- 28.Le N-T, et al. Selective oxidation of 5-hydroxymethyl-2-furfural into 2,5-diformylfuran over VO2+ and Cu2+ ions immobilized on sulfonated carbon catalysts. Appl. Catal., A. 2013;464-465:305–312. doi: 10.1016/j.apcata.2013.06.002. [DOI] [Google Scholar]
- 29.Chen J, et al. One-step approach to 2,5-diformylfuran from fructose by proton- and vanadium-containing graphitic carbon nitride. ChemCatChem. 2014;6:3174–3181. doi: 10.1002/cctc.201402323. [DOI] [Google Scholar]
- 30.Ghezali W, et al. choline chloride/DMSO solvent for the direct synthesis of diformylfuran from carbohydrates in the presence of heteropolyacids. Green Chem. 2015;17:4459–4464. doi: 10.1039/C5GC01336D. [DOI] [Google Scholar]
- 31.Gupta NK, Nishimura S, Takagaki A, Ebitani K. Hydrotalcite-supported gold-nanoparticle-catalyzed highly efficient base-free aqueous oxidation of 5-hydroxymethylfurfural into 2, 5-furandicarboxylic acid under atmospheric oxygen pressure. Green Chem. 2011;13:824–827. doi: 10.1039/c0gc00911c. [DOI] [Google Scholar]
- 32.Nguyen CV, et al. A metal-free, high nitrogen-doped nanoporous graphitic carbon catalyst for an effective aerobic HMF-to-FDCA conversion. Green Chem. 2016;18:5957–5961. doi: 10.1039/C6GC02118B. [DOI] [Google Scholar]
- 33.Jorge EYC, et al. Metal-exchanged magnetic β-zeolites: valorization of lignocellulosic biomass-derived compounds to platform chemicals. Green Chem. 2017;19:3856–3868. doi: 10.1039/C7GC01178D. [DOI] [Google Scholar]
- 34.Lima TM, et al. Magnetic ZSM-5 zeolite: a selective catalyst for the valorization of furfuryl alcohol to γ-valerolactone, alkyl levulinates or levulinic acid. Green Chem. 2016;18:5586–5593. doi: 10.1039/C6GC01296E. [DOI] [Google Scholar]
- 35.Tadele K, Verma S, Gonzalez MA, Varma RS. A sustainable approach to empower the bio-based future: upgrading of biomass via process intensification. Green Chem. 2017;19:1624–1627. doi: 10.1039/C6GC03568J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verma S, et al. Sustainable pathway to furanics from biomass via heterogeneous organo-catalysis. Green Chem. 2017;19:164–168. doi: 10.1039/C6GC02551J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verma S, Baig RB, Nadagouda MN, Varma RS. Sustainable strategy utilizing biomass: visible‐light‐mediated synthesis of γ‐valerolactone. ChemCatChem. 2016;8:690–693. doi: 10.1002/cctc.201501352. [DOI] [Google Scholar]
- 38.Verma S, Baig RB, Nadagouda MN, Varma RS. Visible light mediated upgrading of biomass to biofuel. Green Chem. 2016;18:1327–1331. doi: 10.1039/C5GC02951A. [DOI] [Google Scholar]
- 39.Varma RS. Greener and sustainable trends in synthesis of organics and nanomaterials. ACS Sustainable Chem. Eng. 2016;4:5866–5878. doi: 10.1021/acssuschemeng.6b01623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Afolabi AS, Abdulkareem AS, Iyuke SE. & van Zyl Pienaar, H. C. Continuous production of carbon nanotubes and diamond films by swirled floating catalyst chemical vapour deposition method. S. Afr. J. Sci. 2009;105:278–281. [Google Scholar]
- 41.Watanabe H, et al. Nitrogen-doped, metal-free activated carbon catalysts for aerobic oxidation of alcohols. ACS Catal. 2015;5:2886–2894. doi: 10.1021/acscatal.5b00375. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.